Spontaneous formation of gold nanostructures in aqueous microdroplets
- PMID: 29674623
- PMCID: PMC5908806
- DOI: 10.1038/s41467-018-04023-z
Spontaneous formation of gold nanostructures in aqueous microdroplets
Abstract
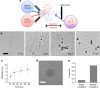
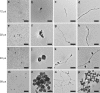
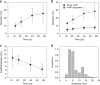
The synthesis of gold nanostructures has received widespread attention owing to many important applications. We report the accelerated synthesis of gold nanoparticles (AuNPs), as well as the reducing-agent-free and template-free synthesis of gold nanoparticles and nanowires in aerosol microdroplets. At first, the AuNP synthesis are carried out by fusing two aqueous microdroplet streams containing chloroauric acid and sodium borohydride. The AuNPs (~7 nm in diameter) are produced within 60 µs at the rate of 0.24 nm µs-1. Compared to bulk solution, microdroplets enhance the size and the growth rate of AuNPs by factors of about 2.1 and 1.2 × 105, respectively. Later, we find that gold nanoparticles and nanowires (~7 nm wide and >2000 nm long) are also formed in microdroplets in the absence of any added reducing agent, template, or externally applied charge. Thus, water microdroplets not only accelerate the synthesis of AuNPs by orders of magnitude, but they also cause spontaneous formation of gold nanostructures.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

