Microtubule dynamics: an interplay of biochemistry and mechanics
- PMID: 29674711
- PMCID: PMC6019280
- DOI: 10.1038/s41580-018-0009-y
Microtubule dynamics: an interplay of biochemistry and mechanics
Abstract
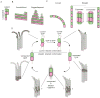
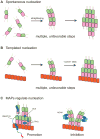
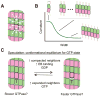
Microtubules are dynamic polymers of αβ-tubulin that are essential for intracellular organization, organelle trafficking and chromosome segregation. Microtubule growth and shrinkage occur via addition and loss of αβ-tubulin subunits, which are biochemical processes. Dynamic microtubules can also engage in mechanical processes, such as exerting forces by pushing or pulling against a load. Recent advances at the intersection of biochemistry and mechanics have revealed the existence of multiple conformations of αβ-tubulin subunits and their central role in dictating the mechanisms of microtubule dynamics and force generation. It has become apparent that microtubule-associated proteins (MAPs) selectively target specific tubulin conformations to regulate microtubule dynamics, and mechanical forces can also influence microtubule dynamics by altering the balance of tubulin conformations. Importantly, the conformational states of tubulin dimers are likely to be coupled throughout the lattice: the conformation of one dimer can influence the conformation of its nearest neighbours, and this effect can propagate over longer distances. This coupling provides a long-range mechanism by which MAPs and forces can modulate microtubule growth and shrinkage. These findings provide evidence that the interplay between biochemistry and mechanics is essential for the cellular functions of microtubules.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

