Adverse drug reactions at adverse drug reaction monitoring center in Raipur: Analysis of spontaneous reports during 1 year
- PMID: 29674797
- PMCID: PMC5892024
- DOI: 10.4103/ijp.IJP_781_16
Adverse drug reactions at adverse drug reaction monitoring center in Raipur: Analysis of spontaneous reports during 1 year
Abstract
Background: India is a developing country and adverse drug reactions (ADRs) influence most of the diseases in our population, and monitoring is required due to the paucity of ADRs. The present study was done to analyze the ADRs at the ADR monitoring center (AMC) of tertiary care hospital in Raipur during 1 year.
Materials and methods: Study of ADR monitoring of outpatient and inpatient was a prospective and observational study carried out between September 2015 and August 2016. The ADRs in the form of Individual Case Safety Report (ICSR) was sent to the Indian database (Vigiflow®).
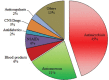
Results: Total ICSRs reported to Vigiflow® were 232 during 1 year. Among them, 63.79% were found to be nonserious and 36.21% were serious. Nearly 45% of ADRs were implicated only due to antimicrobials, which is highest among all other groups of drugs. A maximum number of ADRs were observed in 31-60 years of age group (52.15%). In causality assessment, the probable cases had a higher incidence (67.24%), followed by possible (27.58%) and certain (4.74%). The frequency of ADR reporting at our AMC was low (0.043%) compared to national average. Our AMC shared 0.35% of total ICSRs, which is insignificant (P < 0.001) compared to the JSS, Mysore and PGIMER, Chandigarh, AMCs, which have shared most of the ICSRs in Vigiflow®.
Conclusions: The frequencies of ADRs reporting in our study are less compared to those reported with other similar studies. Underreporting is a very serious concern in Raipur, and Pharmacovigilance Programme of India must intercede to pick up ADRs across the country.
Keywords: Adverse drug reaction monitoring center; Pharmacovigilance Programme of India; Vigiflow®; adverse drug reactions; individual case safety reports.
Conflict of interest statement
There are no conflicts of interest.
Figures



References
-
- Vivekanandan K, Thota P, Janarthanan VV, Singh GN. Pharmacovigilante's in the pharmacovigilance programme of India: Ideal qualities and skills. J Young Pharm. 2016;8:291–2.
-
- van Hunsel F, Härmark L, Pal S, Olsson S, van Grootheest K. Experiences with adverse drug reaction reporting by patients: An 11-country survey. Drug Saf. 2012;35:45–60. - PubMed
-
- Mittal N, Mittal R, Gupta MC. An overview of the pharmacovigilance system in India. Clin Res Regul Aff. 2016;33:4–8.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

