Role of microRNAs and Exosomes in Helicobacter pylori and Epstein-Barr Virus Associated Gastric Cancers
- PMID: 29675003
- PMCID: PMC5895734
- DOI: 10.3389/fmicb.2018.00636
Role of microRNAs and Exosomes in Helicobacter pylori and Epstein-Barr Virus Associated Gastric Cancers
Abstract
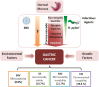
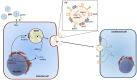
Emerging evidence suggests that chronic inflammation caused by pathogen infection is connected to the development of various types of cancer. It is estimated that up to 20% of all cancer deaths is linked to infections and inflammation. In gastric cancer, such triggers can be infection of the gastric epithelium by either Helicobacter pylori (H. pylori), a bacterium present in half of the world population; or by Epstein-Barr virus (EBV), a double-stranded DNA virus which has recently been associated with gastric cancer. Both agents can establish lifelong inflammation by evolving to escape immune surveillance and, under certain conditions, contribute to the development of gastric cancer. Non-coding RNAs, mainly microRNAs (miRNAs), influence the host innate and adaptive immune responses, though long non-coding RNAs and viral miRNAs also alter these processes. Reports suggest that chronic infection results in altered expression of host miRNAs. In turn, dysregulated miRNAs modulate the host inflammatory immune response, favoring bacterial survival and persistence within the gastric mucosa. Given the established roles of miRNAs in tumorigenesis and innate immunity, they may serve as an important link between H. pylori- and EBV-associated inflammation and carcinogenesis. Example of this is up-regulation of miR-155 in H. pylori and EBV infection. The tumor environment contains a variety of cells that need to communicate with each other. Extracellular vesicles, especially exosomes, allow these cells to deliver certain type of information to other cells promoting cancer growth and metastasis. Exosomes have been shown to deliver not only various types of genetic information, mainly miRNAs, but also cytotoxin-associated gene A (CagA), a major H. pylori virulence factor. In addition, a growing body of evidence demonstrates that exosomes contain genetic material of viruses and viral miRNAs and proteins such as EBV latent membrane protein 1 (LMP1) which are delivered into recipient cells. In this review, we focus on the dysregulated H. pylori- and EBV-associated miRNAs while trying to unveil possible causal mechanisms. Moreover, we discuss the role of exosomes as vehicles for miRNA delivery in H. pylori- and EBV-related carcinogenesis.
Keywords: Epstein-Barr virus; Exosomes; Helicobacter pylori; gastric cancer; lncRNA (long non-coding RNA); microRNA.
Figures
References
-
- Alarcón A., Figueroa U., Espinoza B., Sandoval A., Carrasco-Aviño G., Aguayo F. R., et al. (2017). Epstein-barr virus–associated gastric carcinoma: the americas' perspective, in Gastric Cancer, eds Mozsik G., Karádi O. (InTech), 10.5772/intechopen.70201 Available online at: https://mts.intechopen.com/books/gastric-cancer/epstein-barr-virus-assoc... - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

