Enhanced AKT Phosphorylation of Circulating B Cells in Patients With Activated PI3Kδ Syndrome
- PMID: 29675019
- PMCID: PMC5895775
- DOI: 10.3389/fimmu.2018.00568
Enhanced AKT Phosphorylation of Circulating B Cells in Patients With Activated PI3Kδ Syndrome
Abstract
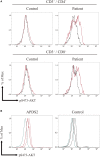
Activated PI3Kδ syndrome (APDS) is a primary immunodeficiency characterized by recurrent respiratory tract infections, lymphoproliferation, and defective IgG production. Heterozygous mutations in PIK3CD, PIK3R1, or PTEN, which are related to the hyperactive phosphoinositide 3-kinase (PI3K) signaling, were recently presented to cause APDS1 or APDS2 (APDSs), or APDS-like (APDS-L) disorder. In this study, we examined the AKT phosphorylation of peripheral blood lymphocytes and monocytes in patients with APDSs and APDS-L by using flow cytometry. CD19+ B cells of peripheral blood in APDS2 patients showed the enhanced phosphorylation of AKT at Ser473 (pAKT) without any specific stimulation. The enhanced pAKT in CD19+ B cells was normalized by the addition of a p110δ inhibitor. In contrast, CD3+ T cells and CD14+ monocytes did not show the enhanced pAKT in the absence of stimulation. These findings were similarly observed in patients with APDS1 and APDS-L. Among CD19+ B cells, enhanced pAKT was prominently detected in CD10+ immature B cells compared with CD10- mature B cells. Enhanced pAKT was not observed in B cells of healthy controls, patients with common variable immunodeficiency, and hyper IgM syndrome due to CD40L deficiency. These results suggest that the enhanced pAKT in circulating B cells may be useful for the discrimination of APDS1, APDS2, and APDS-L from other antibody deficiencies.
Keywords: AKT phosphorylation; activated PI3 kinase delta syndrome; catalytic subunit p110δ of phosphatidylinositol 3-kinase; flow cytometry; immunodeficiency; regulatory subunit p85α of phosphatidylinositol 3-kinase.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

