Impact of Paneth Cell Autophagy on Inflammatory Bowel Disease
- PMID: 29675025
- PMCID: PMC5895641
- DOI: 10.3389/fimmu.2018.00693
Impact of Paneth Cell Autophagy on Inflammatory Bowel Disease
Abstract
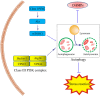
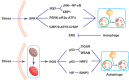
Intestinal mucosal barrier, mainly consisting of the mucus layer and epithelium, functions in absorbing nutrition as well as prevention of the invasion of pathogenic microorganisms. Paneth cell, an important component of mucosal barrier, plays a vital role in maintaining the intestinal homeostasis by producing antimicrobial materials and controlling the host-commensal balance. Current evidence shows that the dysfunction of intestinal mucosal barrier, especially Paneth cell, participates in the onset and progression of inflammatory bowel disease (IBD). Autophagy, a cellular stress response, involves various physiological processes, such as secretion of proteins, production of antimicrobial peptides, and degradation of aberrant organelles or proteins. In the recent years, the roles of autophagy in the pathogenesis of IBD have been increasingly studied. Here in this review, we mainly focus on describing the roles of Paneth cell autophagy in IBD as well as several popular autophagy-related genetic variants in Penath cell and the related therapeutic strategies against IBD.
Keywords: Paneth cell; autophagy; endoplasmic reticulum stress; inflammatory bowel disease; unfolded protein response.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

