Phase I/II clinical trial of everolimus combined with gemcitabine/cisplatin for metastatic triple-negative breast cancer
- PMID: 29675095
- PMCID: PMC5907662
- DOI: 10.7150/jca.24035
Phase I/II clinical trial of everolimus combined with gemcitabine/cisplatin for metastatic triple-negative breast cancer
Abstract
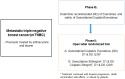
Background: The PI3K/AKT/mTOR pathway is an important oncogenic driver in triple-negative breast cancer (TNBC). This study investigated the clinical efficacy and safety of the combination of gemcitabine and cisplatin with everolimus (GPE) in patients with metastatic TNBC. Methods: In phase I, we assessed the maximum tolerated dose (MTD) of GPE in metastatic TNBC patients. Then, using a seamless design, we conducted a randomized phase II trial to compare GPE to GP in terms of progression-free survival (PFS) and toxicity. In addition, we investigated the mutational status of PIK3CA (E542K, E545K, H1047R) in tumor tissues (n=14) and cell-free DNA (cfDNA) from blood samples (n=23) using droplet digital PCR. Results: In phase I (n=9), we found that the MTD of GPE was gemcitabine 800 mg/m2 and cisplatin 30 mg/m2 on days 1 and 8 every 3 weeks along with everolimus 5 mg daily. Phase II was terminated early after 14 patients had been enrolled because of slow recruitment and concerns about efficacy. Results of the combined analysis of phases I and II showed the objective response rate (ORR) of GPE (n=16) was 31.3% and the median PFS was 5.5 months (95% CI, 3.5-7.5). Stomatitis and hematologic toxicities were observed most frequently in the GPE arm. PIK3CA mutations were identified in 8 (57.1%) tumor samples and 17 (73.9%) cfDNA samples; there was no significant association between PIK3CA mutation status and response to GPE treatment. Conclusions: Although the majority of patients with metastatic TNBC demonstrated PIK3CA mutations in cfDNA, the addition of everolimus to gemcitabine/cisplatin did not have a synergistic effect in these patients. Further studies are needed to determine the most effective way to target the PI3K/AKT/mTOR pathway in TNBC patients.
Keywords: PIK3CA mutation; Triple negative breast cancer; cell free DNA; mTOR inhibitor.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
A Randomized Phase II Neoadjuvant Study of Cisplatin, Paclitaxel With or Without Everolimus in Patients with Stage II/III Triple-Negative Breast Cancer (TNBC): Responses and Long-term Outcome Correlated with Increased Frequency of DNA Damage Response Gene Mutations, TNBC Subtype, AR Status, and Ki67.Clin Cancer Res. 2017 Aug 1;23(15):4035-4045. doi: 10.1158/1078-0432.CCR-16-3055. Epub 2017 Mar 7. Clin Cancer Res. 2017. PMID: 28270498 Free PMC article. Clinical Trial.
-
Correlation between PIK3CA mutations in cell-free DNA and everolimus efficacy in HR+, HER2- advanced breast cancer: results from BOLERO-2.Br J Cancer. 2017 Mar 14;116(6):726-730. doi: 10.1038/bjc.2017.25. Epub 2017 Feb 9. Br J Cancer. 2017. PMID: 28183140 Free PMC article. Clinical Trial.
-
Phase I Study of Everolimus in Combination with Gemcitabine and Split-Dose Cisplatin in Advanced Urothelial Carcinoma.Bladder Cancer. 2016 Jan 7;2(1):111-117. doi: 10.3233/BLC-150038. Bladder Cancer. 2016. PMID: 27376132 Free PMC article.
-
Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: a review.Breast Cancer Res Treat. 2018 Jun;169(3):397-406. doi: 10.1007/s10549-018-4697-y. Epub 2018 Feb 7. Breast Cancer Res Treat. 2018. PMID: 29417298 Review.
-
SABCS 2016: systemic therapy for metastatic breast cancer.Memo. 2017;10(2):86-89. doi: 10.1007/s12254-017-0326-4. Epub 2017 Apr 4. Memo. 2017. PMID: 28725277 Free PMC article. Review.
Cited by
-
Everolimus inhibits the proliferation and migration of epidermal growth factor receptor-resistant lung cancer cells A549 via regulating the microRNA-4328/phosphatase and tensin homolog signaling pathway.Oncol Lett. 2019 Nov;18(5):5269-5276. doi: 10.3892/ol.2019.10887. Epub 2019 Sep 19. Oncol Lett. 2019. Retraction in: Oncol Lett. 2025 Jun 10;30(2):388. doi: 10.3892/ol.2025.15133. PMID: 31612036 Free PMC article. Retracted.
-
The PI3K/Akt/mTOR Signaling Pathway in Triple-Negative Breast Cancer: A Resistance Pathway and a Prime Target for Targeted Therapies.Cancers (Basel). 2025 Jul 3;17(13):2232. doi: 10.3390/cancers17132232. Cancers (Basel). 2025. PMID: 40647529 Free PMC article. Review.
-
Detection of phenotype-specific therapeutic vulnerabilities in breast cells using a CRISPR loss-of-function screen.Mol Oncol. 2021 Aug;15(8):2026-2045. doi: 10.1002/1878-0261.12951. Epub 2021 May 1. Mol Oncol. 2021. PMID: 33759347 Free PMC article.
-
The Role of mTORC1 Pathway and Autophagy in Resistance to Platinum-Based Chemotherapeutics.Int J Mol Sci. 2023 Jun 26;24(13):10651. doi: 10.3390/ijms241310651. Int J Mol Sci. 2023. PMID: 37445831 Free PMC article. Review.
-
Advances in the Detection Technologies and Clinical Applications of Circulating Tumor DNA in Metastatic Breast Cancer.Cancer Manag Res. 2020 May 18;12:3547-3560. doi: 10.2147/CMAR.S249041. eCollection 2020. Cancer Manag Res. 2020. PMID: 32547192 Free PMC article. Review.
References
-
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA. et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. - PubMed
-
- Thike AA, Cheok PY, Jara-Lazaro AR, Tan B, Tan P, Tan PH. Triple-negative breast cancer: clinicopathological characteristics and relationship with basal-like breast cancer. Mod Pathol. 2010;23(1):123–33. - PubMed
-
- Oakman C, Viale G, Di Leo A. Management of triple negative breast cancer. Breast. 2010;19(5):312–21. - PubMed
-
- Maisano R, Zavettieri M, Azzarello D, Raffaele M, Maisano M, Bottari M, Nardi M. Carboplatin and gemcitabine combination in metastatic triple-negative anthracycline- and taxane-pretreated breast cancer patients: a phase II study. J Chemother. 2011;23(1):40–3. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous