The aromatic dianion metalloles
- PMID: 29675144
- PMCID: PMC5883866
- DOI: 10.1039/c7sc04454b
The aromatic dianion metalloles
Abstract
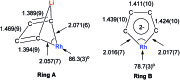
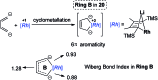
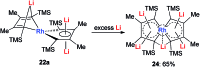
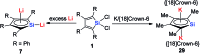
Metalloaromatic species are unique and important both experimentally and theoretically. Significant progress has been made during the past few decades. New aromatic systems have challenged and extended the concept of aromaticity remarkably. In this perspective, recent results on the study of the dianion aromatic metalloles and their corresponding analogues are reviewed. These include the dilithio group 14 metalloles, group 13 metalloles and transition metal metalloles. X-ray crystallography has made a key contribution to the understanding of the structures. Various theoretical tools, such as NICS and AdNDP, make it possible to measure the aromaticity beyond Hückel's rule. The dianion butadiene skeletons play a key role in these metalloles and can be regarded as non-innocent ligands, which accept the electrons from the metal center and thus form the aromatic rings. By simply changing the central metals to different metals, the metallole analogues such as dicupra[10]annulenes and spiroaromatic palladoles can also be generated, which opens a door to synthesize other metalla-macrocyclic aromatics. Key challenges and envisioned opportunities for the future, such as applying these dianion metalloles as novel ligands of transition metals and generating new types of organometallic aromatic system, are also discussed.
Figures
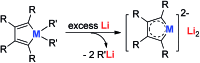



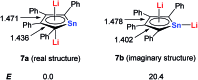





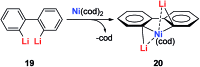








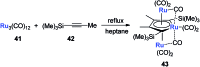

References
-
- Faraday M. Philos. Trans. R. Soc. London. 1825;115:440–446.
-
- Boldyrev A. I., Wang L.-S. Chem. Rev. 2005;105:3716–3757. - PubMed
- Tsipis C. A. Coord. Chem. Rev. 2005;249:2740–2762.
- Zubarev D. Yu., Averkiev B. B., Zhai H.-J., Wang L. S., Boldyrev A. I. Phys. Chem. Chem. Phys. 2008;10:257–267. - PubMed
- Feixas F., Matito E., Poater J., Solà M. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2013;3:105–122.
-
- Bleeke J. R. Chem. Rev. 2001;101:1205–1228. - PubMed
- Jia G. Acc. Chem. Res. 2004;37:479–486. - PubMed
- Wright L. J. Dalton Trans. 2006:1821–1827. - PubMed
- Landorf C. W., Haley M. M. Angew. Chem., Int. Ed. 2006;45:3914–3936. - PubMed
- Chen J., Jia G. Coord. Chem. Rev. 2013;257:2491–2521.
- Frogley B. J., Wright L. J. Coord. Chem. Rev. 2014;270–271:151–166.
- Cao X.-Y., Zhao Q., Lin Z., Xia H. Acc. Chem. Res. 2014;47:341–354. - PubMed
- Roy S., Rosenthal U., Jemmis E. D. Acc. Chem. Res. 2014;47:2917–2930. - PubMed
- Frogley B. J. and Wright L. J., Chem.–Eur. J., 10.1002/chem.201704888. - DOI
-
- Thorn D. L., Hoffmann R. Nouv. J. Chim. 1979;3:39–45.
-
- Elliott G. P., Roper W. R., Waters J. M. J. Chem. Soc., Chem. Commun. 1982:811–813.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

