Alkylative kinetic resolution of vicinal diols under phase-transfer conditions: a chiral ammonium borinate catalysis
- PMID: 29675168
- PMCID: PMC5885781
- DOI: 10.1039/c7sc04854h
Alkylative kinetic resolution of vicinal diols under phase-transfer conditions: a chiral ammonium borinate catalysis
Abstract
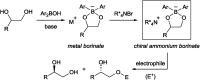
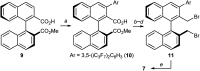
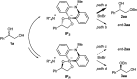
Herein, we report the first alkylative kinetic resolution of vicinal alcohols realized by cooperative use of a chiral quaternary ammonium salt and an achiral borinic acid. In addition, a catalytic regioselective alkylation of a secondary alcohol in the presence of an unprotected primary one is presented, emphasizing the unique selectivity and potential of this ammonium borinate catalysis.
Figures
Similar articles
-
Highly enantioselective phase-transfer-catalyzed alkylation of protected alpha-amino acid amides toward practical asymmetric synthesis of vicinal diamines, alpha-amino ketones, and alpha-amino alcohols.J Am Chem Soc. 2005 Apr 13;127(14):5073-83. doi: 10.1021/ja0459328. J Am Chem Soc. 2005. PMID: 15810842
-
Synthesis of a C2-Symmetric Chiral Borinic Acid and Its Application in Catalytic Desymmetrization of 2,2-Disubstituted-1,3-Propanediols.J Am Chem Soc. 2023 Apr 10. doi: 10.1021/jacs.3c02331. Online ahead of print. J Am Chem Soc. 2023. PMID: 37036443
-
Regioselective, borinic acid-catalyzed monoacylation, sulfonylation and alkylation of diols and carbohydrates: expansion of substrate scope and mechanistic studies.J Am Chem Soc. 2012 May 16;134(19):8260-7. doi: 10.1021/ja302549c. Epub 2012 Apr 25. J Am Chem Soc. 2012. PMID: 22533533
-
Advances in asymmetric oxidative kinetic resolution of racemic secondary alcohols catalyzed by chiral Mn(III) salen complexes.Chirality. 2017 Dec;29(12):798-810. doi: 10.1002/chir.22768. Epub 2017 Sep 29. Chirality. 2017. PMID: 28963733 Review.
-
Recent Progress in Asymmetric Ion-Pairing Catalysis with Ammonium Salts.Chemistry. 2019 Mar 12;25(15):3740-3751. doi: 10.1002/chem.201803752. Epub 2019 Jan 11. Chemistry. 2019. PMID: 30358913 Review.
Cited by
-
Chemical approaches to the sulfation of small molecules: current progress and future directions.Essays Biochem. 2024 Dec 4;68(4):449-466. doi: 10.1042/EBC20240001. Essays Biochem. 2024. PMID: 38958528 Free PMC article. Review.
-
Enantioselective Kinetic Resolution/Desymmetrization of Para-Quinols: A Case Study in Boronic-Acid-Directed Phosphoric Acid Catalysis.Adv Synth Catal. 2020 Jan 23;362(2):295-301. doi: 10.1002/adsc.201900816. Epub 2019 Sep 6. Adv Synth Catal. 2020. PMID: 34093103 Free PMC article.
-
Asymmetric phase-transfer catalysis.Nat Rev Chem. 2024 Nov;8(11):851-869. doi: 10.1038/s41570-024-00642-x. Epub 2024 Oct 9. Nat Rev Chem. 2024. PMID: 39385042 Review.
-
Kinetic resolution of racemic tertiary allylic alcohols through SN2' reaction using a chiral bisphosphoric acid/silver(i) salt co-catalyst system.Chem Sci. 2022 Jul 20;13(33):9607-9613. doi: 10.1039/d2sc03052g. eCollection 2022 Aug 24. Chem Sci. 2022. PMID: 36091917 Free PMC article.
References
-
- Freedman H. H., Dubois R. A. Tetrahedron Lett. 1975;16:3251.
- de la Zerda J., Barak G., Sasson Y. Tetrahedron. 1989;45:1533.
- Liu Y., Ciszewski L., Shen L., Prashad M. Org. Process Res. Dev. 2014;18:1142.
-
- For chiral phase-transfer catalyzed O-alkylation of enolates, see; Jolliffe J. D., Armstrong R. J., Smith M. D., Nat. Chem., 2017, 9 , 558 . - PubMed
-
- Chan L., Taylor M. S. Org. Lett. 2011;13:3090. - PubMed
- Lee D., Williamson C. L., Chan L., Taylor M. S. J. Am. Chem. Soc. 2012;134:8260. - PubMed
- Dimitrijevic E., Taylor M. S. Chem. Sci. 2013;4:3298.
- D'Angelo K. A., Taylor M. S. J. Am. Chem. Soc. 2016;138:11058. - PubMed
- D'Angelo K. A., Taylor M. S. Chem. Commun. 2017;53:5978. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

