Stereoselective cobalt-catalyzed halofluoroalkylation of alkynes
- PMID: 29675224
- PMCID: PMC5892352
- DOI: 10.1039/c7sc04916a
Stereoselective cobalt-catalyzed halofluoroalkylation of alkynes
Abstract
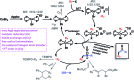
Stereoselective additions of highly functionalized reagents to available unsaturated hydrocarbons are an attractive synthetic tool due to their high atom economy, modularity, and rapid generation of complexity. We report efficient cobalt-catalyzed (E)-halofluoroalkylations of alkynes/alkenes that enable the construction of densely functionalized, stereodefined fluorinated hydrocarbons. The mild conditions (2 mol% cat., 20 °C, acetone/water, 3 h) tolerate various functional groups, i.e. halides, alcohols, aldehydes, nitriles, esters, and heteroarenes. This reaction is the first example of a highly stereoselective cobalt-catalyzed halo-fluoroalkylation. Unlike related cobalt-catalyzed reductive couplings and Heck-type reactions, it operates via a radical chain mechanism involving terminal halogen atom transfer which obviates the need for a stoichiometric sacrificial reductant.
Figures









References
-
- Müller K., Faeh C., Diederich F. Science. 2007;317:1881. - PubMed
- Hagmann W. K. J. Med. Chem. 2008;51:4359. - PubMed
- O'Hagan D. Chem. Soc. Rev. 2008;37:308.
- Purser S., Moore P. R., Swallow S., Gouverneur V. Chem. Soc. Rev. 2008;37:320. - PubMed
- Gouverneur V. and Müller K., Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications, Imperial College Press, London, 2012.
-
-
Trifluoromethylations:
- Furuya T., Kamlet A. S., Ritter T. Nature. 2011;473:470. - PMC - PubMed
- Tomashenko O. A., Grushin V. V. Chem. Rev. 2011;111:4475. - PubMed
- Besset T., Schneider C., Cahard D. Angew. Chem., Int. Ed. 2012;51:5048. - PubMed
- Liang T., Neumann C. N., Ritter T. Angew. Chem., Int. Ed. 2013;52:8214. - PubMed
- Chu L., Qing F.-L. Acc. Chem. Res. 2014;47:1513. - PubMed
- Merino E., Nevado C. Chem. Soc. Rev. 2014;43:6598. - PMC - PubMed
- Egami H., Sodeoka M. Angew. Chem., Int. Ed. 2014;53:8294. - PubMed
- Charpentier J., Früh N., Togni A. Chem. Rev. 2015;115:650. - PubMed
- Liu X., Xu C., Wang M., Liu Q. Chem. Rev. 2015;115:683. - PubMed
- Ni C., Hu M., Hu J. Chem. Rev. 2015;115:765. - PubMed
- Yang X., Wu T., Phipps R. J., Toste F. D. Chem. Rev. 2015;115:826. - PMC - PubMed
- Alonso C., Marigotra E. M., Rubiales G., Palacios F. Chem. Rev. 2015;115:1847. - PubMed
- Gao P., Song X.-R., Liu X.-Y., Liang Y.-M. Chem.–Eur. J. 2015;21:7648. - PubMed
-
-
-
Difluoroalkylations:
- Fujikawa K., Fujioka Y., Kobayashi A., Amii H. Org. Lett. 2011;13:5560. - PubMed
- Fier P. S., Hartwig J. F. J. Am. Chem. Soc. 2012;134:5524. - PMC - PubMed
- He Z., Luo T., Hu M., Cao Y., Hu J. Angew. Chem., Int. Ed. 2012;51:3944. - PubMed
- Qi Q., Shen Q., Lu L. J. Am. Chem. Soc. 2012;134:6548. - PubMed
- Feng Z., Chen F., Zhang X. Org. Lett. 2012;14:1938. - PubMed
- Jiang X., Liu L., Qing F.-L. Org. Lett. 2012;14:2870. - PubMed
- He Z., Hu M., Luo T., Li L., Hu J. Angew. Chem., Int. Ed. 2012;51:11545. - PubMed
- Prakash G. K. S., Ganesh S. K., Jones J.-P., Kulkarni A., Masood K., Swabeck J. K., Olah G. A. Angew. Chem., Int. Ed. 2012;51:12090. - PubMed
- Li Z., Cui Z., Liu Z.-Q. Org. Lett. 2013;15:406. - PubMed
- Min Q.-Q., Yin Z., Feng Z., Guo W.-H., Zhang X. J. Am. Chem. Soc. 2014;136:1230. - PubMed
- Feng Z., Xiao Y.-L., Zhang X. Org. Chem. Front. 2014;1:11.
- Xiao Y.-L., Guo W.-H., He G.-Z., Pan Q., Zhang X. Angew. Chem., Int. Ed. 2014;53:9909. - PubMed
- Ge S., Arlow S. I., Mormino M. G., Hartwig J. F. J. Am. Chem. Soc. 2014;136:14401. - PMC - PubMed
- Feng Z., Min Q.-Q., Zhao H.-Y., Gu J.-W., Zhang X. Angew. Chem., Int. Ed. 2015;54:1270. - PubMed
- Shao C., Shi G., Zhang Y., Pan S., Guan X. Org. Lett. 2015;17:2652. - PubMed
- An L., Xiao Y.-L., Min Q.-Q., Zhang X. Angew. Chem., Int. Ed. 2015;54:9079. - PubMed
- Li Z., Garcia-Domínguez A., Nevado C. J. Am. Chem. Soc. 2015;137:11610. - PubMed
- Li G., Wang T., Fei F., Su Y.-M., Li Y., Lan Q., Wang X.-S. Angew. Chem., Int. Ed. 2016;55:3491. - PubMed
- Xiao Y.-L., Min Q.-Q., Xu C., Wang R.-W., Zhang X. Angew. Chem., Int. Ed. 2016;55:5837. - PubMed
- Ivanova M. V., Bayle A., Besset T., Poisson T., Pannecoucke X. Angew. Chem., Int. Ed. 2015;54:13406. - PubMed
- Wang X., Studer A. Org. Lett. 2017;19:2977. - PMC - PubMed
- Besset T., Poisson T., Pannecoucke X. Eur. J. Org. Chem. 2015:2765.
- Belhomme M.-C., Besset T., Poisson T., Pannecoucke X. Chem.–Eur. J. 2015;21:12836. - PubMed
-
-
-
. For selected other methods, see:
- Takeyama Y., Ichinose Y., Oshima K., Utimato K. Tetrahedron Lett. 1989;30:3159.
- Li Y., Li H., Hu J. Tetrahedron. 2009;65:478.
- Fang X., Yang X., Yang X., Mao S., Wang Y., Chen G., Wu F. Tetrahedron. 2007;63:10684.
- Huang W., Lv L., Zhang Y. Chin. J. Chem. 1990;4:350.
- Rong G.-B., Keese R. Tetrahedron Lett. 1990;31:5615.
- Tsuchii K., Imura M., Kamada N., Hirao T., Ogawa A. J. Org. Chem. 2004;69:6658. - PubMed
- Ishihara T., Kuroboshi M., Okada Y. Chem. Lett. 1986:1895.
- Ma S., Lu X. Tetrahedron. 1990;46:357.
- Jennings M. P., Cork E. A., Ramachandran P. V. J. Org. Chem. 2000;65:8763. - PubMed
- Motoda D., Kinoshita H., Shinokubo H., Oshima K. Adv. Synth. Catal. 2002;344:261.
-
-
- Xu T., Cheung C., Hu X. Angew. Chem., Int. Ed. 2014;53:4910. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

