Discrete Cu(i) complexes for azide-alkyne annulations of small molecules inside mammalian cells
- PMID: 29675241
- PMCID: PMC5892125
- DOI: 10.1039/c7sc04643j
Discrete Cu(i) complexes for azide-alkyne annulations of small molecules inside mammalian cells
Abstract
The archetype reaction of "click" chemistry, namely, the copper-promoted azide-alkyne cycloaddition (CuAAC), has found an impressive number of applications in biological chemistry. However, methods for promoting intermolecular annulations of exogenous, small azides and alkynes in the complex interior of mammalian cells, are essentially unknown. Herein we demonstrate that isolated, well-defined copper(i)-tris(triazolyl) complexes featuring designed ligands can readily enter mammalian cells and promote intracellular CuAAC annulations of small, freely diffusible molecules. In addition to simplifying protocols and avoiding the addition of "non-innocent" reductants, the use of these premade copper complexes leads to more efficient processes than with the alternative, in situ made copper species prepared from Cu(ii) sources, tris(triazole) ligands and sodium ascorbate. Under the reaction conditions, the well-defined copper complexes exhibit very good cell penetration properties, and do not present significant toxicities.
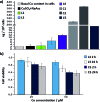
Figures





References
-
- Bleackley M. R., MacGillivray R. T. A. BioMetals. 2011;24:785. - PubMed
- Kennedy D. C., McKay C. S., Legault M. C. B., Danielson D. C., Blake J. A., Pegoraro A. F., Stolow A., Mester Z., Pezacki J. P. J. Am. Chem. Soc. 2011;133:17993. - PubMed
- Teyssot M.-L., Jarrousse A.-S., Chevry A., De H., Beaudoin C., Manin M., Nolan S. P., Díez-González S., Morel L., Gautier A. Chem.–Eur. J. 2009;15:314. - PubMed
-
- Yang M., Li J., Chen P. R. Chem. Soc. Rev. 2014;43:6511. - PubMed
- Chankeshwara S. V., Indrigo E., Bradley M. Curr. Opin. Chem. Biol. 2014;21:128. - PubMed
- Sletten E. M., Bertozzi C. R. Angew. Chem., Int. Ed. 2009;48:6974. - PMC - PubMed
- Patterson D. M., Nazarova L. A., Prescher J. A. ACS Chem. Biol. 2014;9:592. - PubMed
- King M., Wagner A. Bioconjugate Chem. 2014;25:825. - PubMed
- Sasmal P. K., Streu C. N., Meggers E. Chem. Commun. 2013;49:1581. - PubMed
- Borrmann A., van Hest J. C. M. Chem. Sci. 2014;5:2123.
- Völker T., Meggers E. Curr. Opin. Chem. Biol. 2015;25:48. - PubMed
- Li J., Chen P. R. Nat. Chem. Biol. 2016;12:129. - PubMed
- Destito P., Couceiro J. R., Ferreira H., López F., Mascareñas J. L. Angew. Chem., Int. Ed. 2017;56:1. - PMC - PubMed
- Oliveira B. L., Guo Z., Bernardes G. J. L. Chem. Soc. Rev. 2017;43:4895. - PubMed
-
- Li J., Yu J., Zhao J., Wang J., Zheng S., Lin S., Chen L., Yang M., Jia S., Zhang X., Chen P. R. Nat. Chem. 2014;6:352. - PubMed
- Völker T., Dempwolff F., Graumann P. L., Meggers E. Angew. Chem., Int. Ed. 2014;53:10536. - PubMed
- Sánchez M. I., Penas C., Vázquez M. E., Mascareñas J. L. Chem. Sci. 2014;5:1901. - PMC - PubMed
- Streu C., Meggers E. Angew. Chem., Int. Ed. 2006;45:5645. - PubMed
- Wang J., Zheng S., Liu Y., Zhang Z., Lin Z., Li J., Zhang G., Wang X., Li J., Chen P. R. J. Am. Chem. Soc. 2016;138:15118. - PubMed
- Tomás-Gamasa M., Martínez-Calvo M., Couceiro J. R., Mascareñas J. L. Nat. Commun. 2016;7:12538. - PMC - PubMed
- Bose S., Ngo A. H., Do L. H. J. Am. Chem. Soc. 2017;139:8792. - PubMed
- Mascareñas J. L., Martínez-Calvo M. Coord. Chem. Rev. 2018;359C:57.
-
- Rubio-Ruiz B., Weiss J. T., Unciti-Broceta A. J. Med. Chem. 2016;59:9974. - PubMed
- Weiss J. T., Dawson J. C., Macleod K. G., Rybski W., Fraser C., Torres-Sánchez C., Patton E. E., Bradley M., Carragher N. O., Unciti-Broceta A. Nat. Commun. 2014;5:3277. - PMC - PubMed
- Weiss J. T., Carragher N. O., Unciti-Broceta A. Sci. Rep. 2015;5:9329. - PMC - PubMed
-
- Spicer C. D., Triemer T., Davis B. G. J. Am. Chem. Soc. 2012;134:800. - PubMed
- Li N., Lim R. K. V., Edwardraja S., Lin Q. J. Am. Chem. Soc. 2011;133:15316. - PMC - PubMed
- Spicer C. D., Davis B. G. Chem. Commun. 2013;49:2747. - PubMed
- Li J., Lin S., Wang J., Jia S., Yang M., Hao Z., Zhang X., Chen P. R. J. Am. Chem. Soc. 2013;135:7330. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

