New application of phthalocyanine molecules: from photodynamic therapy to photothermal therapy by means of structural regulation rather than formation of aggregates
- PMID: 29675251
- PMCID: PMC5892404
- DOI: 10.1039/c7sc05115h
New application of phthalocyanine molecules: from photodynamic therapy to photothermal therapy by means of structural regulation rather than formation of aggregates
Abstract
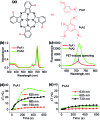
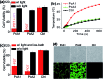
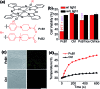
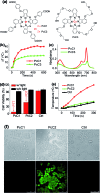
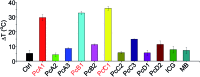
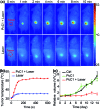
Phthalocyanine (Pc) molecules exhibit high extinction coefficients in near-infrared region, rendering them well-suited for phototherapies, but most of their applications are limited to the field of photodynamic therapy (PDT). Herein, for the first time, we illustrate that Pc molecules can be endowed with excellent photothermal properties by means of structural regulation rather than formation of aggregates. Three representative Pc derivatives show efficient activities of photothermal therapy (PTT) against human hepatocellular carcinoma cells. Among them, copper phthalocyanine (PcC1) exhibits a high in vivo PTT efficacy against mice bearing S180 tumors. The unique investigation in this article should light up a perspective of Pc's new applications for PTT, which enable to make up the inherent defects of PDT.
Figures
Similar articles
-
Tumor microenvironment-responsive nanohybrid for hypoxia amelioration with photodynamic and near-infrared II photothermal combination therapy.Acta Biomater. 2022 Jul 1;146:450-464. doi: 10.1016/j.actbio.2022.04.044. Epub 2022 May 6. Acta Biomater. 2022. PMID: 35526739
-
Hollow silica nanoparticles loaded with hydrophobic phthalocyanine for near-infrared photodynamic and photothermal combination therapy.Biomaterials. 2013 Oct;34(32):7905-12. doi: 10.1016/j.biomaterials.2013.07.027. Epub 2013 Jul 24. Biomaterials. 2013. PMID: 23891514
-
Near-infrared and metal-free tetra(butylamino)phthalocyanine nanoparticles for dual modal cancer phototherapy.RSC Adv. 2020 Jul 9;10(43):25958-25965. doi: 10.1039/d0ra03898a. eCollection 2020 Jul 3. RSC Adv. 2020. PMID: 35518584 Free PMC article.
-
Recent Progress of Copper-Based Nanomaterials in Tumor-Targeted Photothermal Therapy/Photodynamic Therapy.Pharmaceutics. 2023 Sep 7;15(9):2293. doi: 10.3390/pharmaceutics15092293. Pharmaceutics. 2023. PMID: 37765262 Free PMC article. Review.
-
Recent progress in the development of near-infrared organic photothermal and photodynamic nanotherapeutics.Biomater Sci. 2018 Mar 26;6(4):746-765. doi: 10.1039/c7bm01210a. Biomater Sci. 2018. PMID: 29485662 Review.
Cited by
-
Enhancing singlet oxygen generation in semiconducting polymer nanoparticles through fluorescence resonance energy transfer for tumor treatment.Chem Sci. 2019 Apr 11;10(19):5085-5094. doi: 10.1039/c8sc05501g. eCollection 2019 May 21. Chem Sci. 2019. PMID: 31183060 Free PMC article.
-
Recent progress of emitting long-wavelength carbon dots and their merits for visualization tracking, target delivery and theranostics.Theranostics. 2023 May 21;13(9):3064-3102. doi: 10.7150/thno.80579. eCollection 2023. Theranostics. 2023. PMID: 37284447 Free PMC article. Review.
-
Modulating the phototoxicity and selectivity of a porphyrazine towards epidermal tumor cells by coordination with metal ions.Photochem Photobiol Sci. 2024 Sep;23(9):1757-1769. doi: 10.1007/s43630-024-00629-z. Epub 2024 Sep 6. Photochem Photobiol Sci. 2024. PMID: 39242437
-
A cruciform phthalocyanine pentad-based NIR-II photothermal agent for highly efficient tumor ablation.Chem Sci. 2019 Jul 18;10(35):8246-8252. doi: 10.1039/c9sc02674f. eCollection 2019 Sep 21. Chem Sci. 2019. PMID: 31673325 Free PMC article.
-
Phthalocyanine aggregates as semiconductor-like photocatalysts for hypoxic-tumor photodynamic immunotherapy.Nat Commun. 2025 Jan 2;16(1):326. doi: 10.1038/s41467-024-55575-2. Nat Commun. 2025. PMID: 39747902 Free PMC article.
References
-
- Sekkat N., van den Bergh H., Nyokong T., Lange N. Molecules. 2012;17:98–144. - PMC - PubMed
- Jiang Z., Shao J., Yang T., Wang J., Jia L. J. Pharm. Biomed. Anal. 2014;87:98–104. - PubMed
- Li X., Zheng B. D., Peng X. H., Li S. Z., Ying J. W., Zhao Y., Huang J. D., Yoon J. Coord. Chem. Rev. 2017 doi: 10.1016/j.ccr.2017.08.003. - DOI
-
- Dolmans D. E. J. G. J., Fukumura D., Jain R. K. Nat. Rev. Cancer. 2003;3:380–387. - PubMed
- Castano A. P., Mroz P., Hamblin M. R. Nat. Rev. Cancer. 2006;6:535–545. - PMC - PubMed
- Pervaiz S., Olivo M. Clin. Exp. Pharmacol. Physiol. 2006;33:551–556. - PubMed
- Lovell J. F., Liu T. W. B., Chen J., Zheng G. Chem. Rev. 2010;110:2839–2857. - PubMed
-
- Huang Z. Technol. Cancer Res. Treat. 2005;4:283–293. - PMC - PubMed
- Zhang Y., He L., Wu J., Wang K., Wang J., Dai W., Yuan A., Wu J., Hu Y. Biomaterials. 2016;107:23–32. - PubMed
- Wang Y., Lin Y., Zhang H. G., Zhu J. J. Cancer Res. Clin. Oncol. 2016;142:813–821. - PMC - PubMed
- Li X., Zheng B. Y., Ke M. R., Zhang Y., Huang J. D., Yoon J. Theranostics. 2017;7:2746–2756. - PMC - PubMed
-
- Zhou Z., Song J., Nie L., Chen X. Chem. Soc. Rev. 2016;45:6597–6626. - PMC - PubMed
- Turan I. S., Yildiz D., Turksoy A., Gunaydin G., Akkaya E. U. Angew. Chem., Int. Ed. 2016;55:2875–2878. - PubMed
- Kim J., Cho H. R., Jeon H., Kim D., Song C., Lee N., Choi S. H., Hyeon T. J. Am. Chem. Soc. 2017;139:10992–10995. - PubMed
- Jung H. S., Han J., Shi H., Koo S., Singh H., Kim H. J., Sessler J. L., Lee J. Y., Kim J. H., Kim J. S. J. Am. Chem. Soc. 2017;139:7595–7602. - PMC - PubMed
- Kolemen S., Ozdemir T., Lee D., Kim G. M., Karatas T., Yoon J., Akkaya E. U. Angew. Chem., Int. Ed. 2016;55:3603–3610. - PubMed
- Li X., Yu S., Lee D., Kim G., Lee B., Cho Y., Zheng B. Y., Ke M. R., Huang J. D., Nam K. T., Chen X., Yoon J. ACS Nano. 2017 doi: 10.1021/acsnano.7b07809. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

