Free-Floating Mesothelial Cells in Pleural Fluid After Lung Surgery
- PMID: 29675416
- PMCID: PMC5895720
- DOI: 10.3389/fmed.2018.00089
Free-Floating Mesothelial Cells in Pleural Fluid After Lung Surgery
Abstract
Objectives: The mesothelium, the surface layer of the heart, lung, bowel, liver, and tunica vaginalis, is a complex tissue implicated in organ-specific diseases and regenerative biology; however, the mechanism of mesothelial repair after surgical injury is unknown. Previous observations indicated seeding of denuded mesothelium by free-floating mesothelial cells may contribute to mesothelial healing. In this study, we investigated the prevalence of mesothelial cells in pleural fluid during the 7 days following pulmonary surgery.
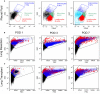
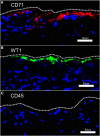
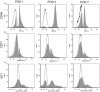
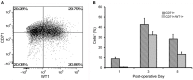
Study design: Flow cytometry was employed to study pleural fluid of 45 patients after lung resection or transplantation. We used histologically validated mesothelial markers (CD71 and WT1) to estimate the prevalence of mesothelial cells.
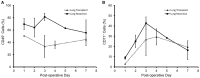
Results: The viability of pleural fluid cells approached 100%. Leukocytes and mesothelial cells were identified in the pleural fluid within the first week after surgery. The leukocyte concentration was relatively stable at all time points. In contrast, mesothelial cells, identified by CD71 and WT1 peaked on POD3. The broad expression of CD71 molecule in postoperative pleural fluid suggests that many of the free-floating non-leukocyte cells were activated or proliferative mesothelial cells.
Conclusion: We demonstrated that pleural fluid post lung surgery is a source of mesothelial cells; most of these cells appear to be viable and, as shown by CD71 staining, activated mesothelial cells. The observed peak of mesothelial cells on POD3 is consistent with a potential reparative role of free-floating mesothelial cells after pulmonary surgery.
Keywords: lung healing; lung regeneration; mesothelial cells; pleural fluid; pneumonectomy.
Figures

Similar articles
-
The pleural mesothelium in development and disease.Front Physiol. 2014 Aug 1;5:284. doi: 10.3389/fphys.2014.00284. eCollection 2014. Front Physiol. 2014. PMID: 25136318 Free PMC article.
-
Evidence for incorporation of free-floating mesothelial cells as a mechanism of serosal healing.J Cell Sci. 2002 Apr 1;115(Pt 7):1383-9. doi: 10.1242/jcs.115.7.1383. J Cell Sci. 2002. PMID: 11896186
-
MCP-1 in pleural injury: CCR2 mediates haptotaxis of pleural mesothelial cells.Am J Physiol Lung Cell Mol Physiol. 2000 Mar;278(3):L591-8. doi: 10.1152/ajplung.2000.278.3.L591. Am J Physiol Lung Cell Mol Physiol. 2000. PMID: 10710532
-
Mesothelial cells from tunica vaginalis, a practical source for mesothelial transplantation.Int J Artif Organs. 2007 Jun;30(6):495-500. doi: 10.1177/039139880703000607. Int J Artif Organs. 2007. PMID: 17628850 Review.
-
Tight junction physiology of pleural mesothelium.Front Physiol. 2014 Jun 24;5:221. doi: 10.3389/fphys.2014.00221. eCollection 2014. Front Physiol. 2014. PMID: 25009499 Free PMC article. Review.
Cited by
-
Active Loading of Pectin Hydrogels for Targeted Drug Delivery.Polymers (Basel). 2022 Dec 26;15(1):92. doi: 10.3390/polym15010092. Polymers (Basel). 2022. PMID: 36616442 Free PMC article.
-
Folliculin haploinsufficiency causes cellular dysfunction of pleural mesothelial cells.Sci Rep. 2021 May 24;11(1):10814. doi: 10.1038/s41598-021-90184-9. Sci Rep. 2021. PMID: 34031471 Free PMC article.
-
FGF2 promotes the expansion of parietal mesothelial progenitor pools and inhibits BMP4-mediated smooth muscle cell differentiation.Front Cell Dev Biol. 2024 Sep 23;12:1387237. doi: 10.3389/fcell.2024.1387237. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39376629 Free PMC article.
-
High Rates of Aseptic Loosening After Revision Total Knee Arthroplasty for Periprosthetic Joint Infection.JB JS Open Access. 2020 Aug 12;5(3):e20.00026. doi: 10.2106/JBJS.OA.20.00026. eCollection 2020 Jul-Sep. JB JS Open Access. 2020. PMID: 32984749 Free PMC article.
-
Post-Surgical Peritoneal Scarring and Key Molecular Mechanisms.Biomolecules. 2021 May 5;11(5):692. doi: 10.3390/biom11050692. Biomolecules. 2021. PMID: 34063089 Free PMC article. Review.
References
-
- Hertzler AE. The Peritoneum. St. Louis: CV Mosby Company; (1919).
-
- Cameron GR, Hassan SM, De SN. Repair of glissons capsule after tangential wounds of the liver. J Pathol Bacteriol (1957) 73:1–10.10.1002/path.1700730102 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

