An MHC-restricted antibody-based chimeric antigen receptor requires TCR-like affinity to maintain antigen specificity
- PMID: 29675462
- PMCID: PMC5904357
- DOI: 10.1038/mto.2016.23
An MHC-restricted antibody-based chimeric antigen receptor requires TCR-like affinity to maintain antigen specificity
Abstract
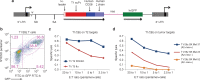
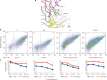
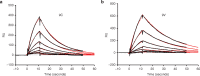
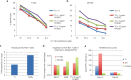
Chimeric antigen receptors (CARs) are synthetic receptors that usually redirect T cells to surface antigens independent of human leukocyte antigen (HLA). Here, we investigated a T cell receptor-like CAR based on an antibody that recognizes HLA-A*0201 presenting a peptide epitope derived from the cancer-testis antigen NY-ESO-1. We hypothesized that this CAR would efficiently redirect transduced T cells in an HLA-restricted, antigen-specific manner. However, we found that despite the specificity of the soluble Fab, the same antibody in the form of a CAR caused moderate lysis of HLA-A2 expressing targets independent of antigen owing to T cell avidity. We hypothesized that lowering the affinity of the CAR for HLA-A2 would improve its specificity. We undertook a rational approach of mutating residues that, in the crystal structure, were predicted to stabilize binding to HLA-A2. We found that one mutation (DN) lowered the affinity of the Fab to T cell receptor-range and restored the epitope specificity of the CAR. DN CAR T cells lysed native tumor targets in vitro, and, in a xenogeneic mouse model implanted with two human melanoma lines (A2+/NYESO+ and A2+/NYESO-), DN CAR T cells specifically migrated to, and delayed progression of, only the HLA-A2+/NY-ESO-1+ melanoma. Thus, although maintaining MHC-restricted antigen specificity required T cell receptor-like affinity that decreased potency, there is exciting potential for CARs to expand their repertoire to include a broad range of intracellular antigens.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

