Involvement of cytosolic and mitochondrial iron in iron overload cardiomyopathy: an update
- PMID: 29675595
- PMCID: PMC6093778
- DOI: 10.1007/s10741-018-9700-5
Involvement of cytosolic and mitochondrial iron in iron overload cardiomyopathy: an update
Abstract
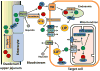
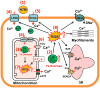
Iron overload cardiomyopathy (IOC) is a major cause of death in patients with diseases associated with chronic anemia such as thalassemia or sickle cell disease after chronic blood transfusions. Associated with iron overload conditions, there is excess free iron that enters cardiomyocytes through both L- and T-type calcium channels thereby resulting in increased reactive oxygen species being generated via Haber-Weiss and Fenton reactions. It is thought that an increase in reactive oxygen species contributes to high morbidity and mortality rates. Recent studies have, however, suggested that it is iron overload in mitochondria that contributes to cellular oxidative stress, mitochondrial damage, cardiac arrhythmias, as well as the development of cardiomyopathy. Iron chelators, antioxidants, and/or calcium channel blockers have been demonstrated to prevent and ameliorate cardiac dysfunction in animal models as well as in patients suffering from cardiac iron overload. Hence, either a mono-therapy or combination therapies with any of the aforementioned agents may serve as a novel treatment in iron-overload patients in the near future. In the present article, we review the mechanisms of cytosolic and/or mitochondrial iron load in the heart which may contribute synergistically or independently to the development of iron-associated cardiomyopathy. We also review available as well as potential future novel treatments.
Keywords: Animal models; Cytosol; Iron overload cardiomyopathy; Mitochondria; Treatment.
Figures
References
-
- Aguilar-Martinez P, Lok CY, Cunat S, Cadet E, Robson K, Rochette J. Juvenile hemochromatosis caused by a novel combination of hemojuvelin G320V/R176C mutations in a 5-year old girl. Haematologica. 2007;92:421–422. - PubMed
-
- Allen KJ, Gurrin LC, Constantine CC, Osborne NJ, Delatycki MB, Nicoll AJ, McLaren CE, Bahlo M, Nisselle AE, Vulpe CD, Anderson GJ, Southey MC, Giles GG, English DR, Hopper JL, Olynyk JK, Powell LW, Gertig DM. Iron-overload-related disease in HFE hereditary hemochromatosis. The New England journal of medicine. 2008;358:221–230. doi: 10.1056/NEJMoa073286. - DOI - PubMed
-
- Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM, Pennell DJ. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. European heart journal. 2001;22:2171–2179. - PubMed
-
- Bartfay WJ, Butany J, Lehotay DC, Sole MJ, Hou D, Bartfay E, Liu PP. A biochemical, histochemical, and electron microscopic study on the effects of iron-loading on the hearts of mice. Cardiovascular pathology: the official journal of the Society for Cardiovascular Pathology. 1999;8:305–314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

