Endoplasmic reticulum stress induces spatial memory deficits by activating GSK-3
- PMID: 29675957
- PMCID: PMC6010738
- DOI: 10.1111/jcmm.13626
Endoplasmic reticulum stress induces spatial memory deficits by activating GSK-3
Abstract
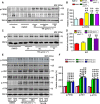
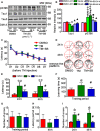
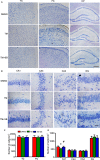
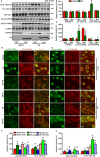
Endoplasmic reticulum (ER) stress is involved in Alzheimer's disease (AD), but the mechanism is not fully understood. Here, we injected tunicamycin (TM), a recognized ER stress inducer, into the brain ventricle of Sprague-Dawley (SD) rats to induce the unfolded protein response (UPR), demonstrated by the enhanced phosphorylation of pancreatic ER kinase (PERK), inositol-requiring enzyme-1 (IRE-1) and activating transcription factor-6 (ATF-6). We observed that UPR induced spatial memory deficits and impairments of synaptic plasticity in the rats. After TM treatment, GSK-3β was activated and phosphorylation of cAMP response element binding protein at Ser129 (pS129-CREB) was increased with an increased nuclear co-localization of pY126-GSK-3β and pS129-CREB. Simultaneous inhibition of GSK-3β by hippocampal infusion of SB216763 (SB) attenuated TM-induced UPR and spatial memory impairment with restoration of pS129-CREB and synaptic plasticity. We concluded that UPR induces AD-like spatial memory deficits with mechanisms involving GSK-3β/pS129-CREB pathway.
Keywords: Alzheimer's disease; cAMP response element binding protein; endoplasmic reticulum; glycogen synthase kinase-3; spatial memory deficits.
© 2018 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures


References
-
- Grundke‐Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule‐associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084‐6089. - PubMed
-
- Huang HC, Jiang ZF. Accumulated amyloid‐beta peptide and hyperphosphorylated tau protein: relationship and links in Alzheimer's disease. J Alzheimers Dis. 2009;16:15‐27. - PubMed
-
- sKo¨pke E, Tung YC, Shaikh S, Alonso AC, Iqbal K, Grundke‐Iqbal I. Microtubule‐associated protein tau. Abnormal phosphorylation of a non‐paired helical filament pool in Alzheimer disease. J Biol Chem 1993;268:24374‐24384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

