Senescent cells re-engineered to express soluble programmed death receptor-1 for inhibiting programmed death receptor-1/programmed death ligand-1 as a vaccination approach against breast cancer
- PMID: 29675979
- PMCID: PMC5989746
- DOI: 10.1111/cas.13618
Senescent cells re-engineered to express soluble programmed death receptor-1 for inhibiting programmed death receptor-1/programmed death ligand-1 as a vaccination approach against breast cancer
Abstract
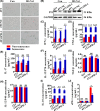
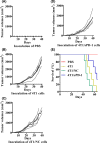
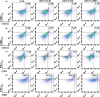
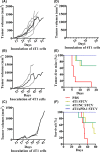
Various types of vaccines have been proposed as approaches for prevention or delay of the onset of cancer by boosting the endogenous immune system. We previously developed a senescent-cell-based vaccine, induced by radiation and veliparib, as a preventive and therapeutic tool against triple-negative breast cancer. However, the programmed death receptor-1/programmed death ligand-1 (PD-1/PD-L1) pathway was found to play an important role in vaccine failure. Hence, we further developed soluble programmed death receptor-1 (sPD1)-expressing senescent cells to overcome PD-L1/PD-1-mediated immune suppression while vaccinating to promote dendritic cell (DC) maturity, thereby amplifying T-cell activation. In the present study, sPD1-expressing senescent cells showed a particularly active status characterized by growth arrest and modified immunostimulatory cytokine secretion in vitro. As expected, sPD1-expressing senescent tumor cell vaccine (STCV/sPD-1) treatment attracted more mature DC and fewer exhausted-PD1+ T cells in vivo. During the course of the vaccine studies, we observed greater safety and efficacy for STCV/sPD-1 than for control treatments. STCV/sPD-1 pre-injections provided complete protection from 4T1 tumor challenge in mice. Additionally, the in vivo therapeutic study of mice with s.c. 4T1 tumor showed that STCV/sPD-1 vaccination delayed tumorigenesis and suppressed tumor progression at early stages. These results showed that STCV/sPD-1 effectively induced a strong antitumor immune response against cancer and suggested that it might be a potential strategy for TNBC prevention.
Keywords: immunotherapy; senescent cell; soluble PD-1; triple-negative breast cancer; vaccine.
© 2018 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures




References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. - PubMed
-
- Rakha EA, Ellis IO. Modern classification of breast cancer: should we stick with morphology or convert to molecular profile characteristics. Adv Anat Pathol. 2011;18:255‐267. - PubMed
-
- Gradishar WJ, Anderson BO, Balassanian R, et al. Invasive Breast Cancer Version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:324‐354. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

