Combination of isocitrate dehydrogenase 1 (IDH1) mutation and podoplanin expression in brain tumors identifies patients at high or low risk of venous thromboembolism
- PMID: 29676036
- PMCID: PMC6099350
- DOI: 10.1111/jth.14129
Combination of isocitrate dehydrogenase 1 (IDH1) mutation and podoplanin expression in brain tumors identifies patients at high or low risk of venous thromboembolism
Abstract
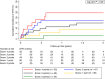
Essentials Risk stratification for venous thromboembolism (VTE) in patients with brain tumors is challenging. Patients with IDH1 wildtype and high podoplanin expression have a 6-month VTE risk of 18.2%. Patients with IDH1 mutation and no podoplanin expression have a 6-month VTE risk of 0%. IDH1 mutation and podoplanin overexpression in primary brain tumors appear to be exclusive.
Summary: Background Venous thromboembolism (VTE) is a frequent complication in primary brain tumor patients. Independent studies revealed that podoplanin expression in brain tumors is associated with increased VTE risk, whereas the isocitrate dehydrogenase 1 (IDH1) mutation is associated with very low VTE risk. Objectives To investigate the interrelation between intratumoral podoplanin expression and IDH1 mutation, and their mutual impact on VTE development. Patients/Methods In a prospective cohort study, intratumoral IDH1 R132H mutation and podoplanin were determined in brain tumor specimens (mainly glioma) by immunohistochemistry. The primary endpoint of the study was symptomatic VTE during a 2-year follow-up. Results All brain tumors that expressed podoplanin to a medium-high extent showed also an IDH1 wild-type status. A score based on IDH1 status and podoplanin expression levels allowed prediction of the risk of VTE. Patients with wild-type IDH1 brain tumors and high podoplanin expression had a significantly increased VTE risk compared with those with mutant IDH1 tumors and no podoplanin expression (6-month risk 18.2% vs. 0%). Conclusions IDH1 mutation and podoplanin overexpression seem to be exclusive. Although brain tumor patients with IDH1 mutation are at very low risk of VTE, the risk of VTE in patients with IDH1 wild-type tumors is strongly linked to podoplanin expression levels.
Keywords: brain neoplasms; cancer; glioma; thromboembolism; thrombosis.
© 2018 International Society on Thrombosis and Haemostasis.
Figures

References
-
- Semrad TJ, O'Donnell R, Wun T, Chew H, Harvey D, Zhou H, White RH. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg 2007; 106: 1121–1127. - PubMed
-
- Riedl J, Preusser M, Nazari PMS, Posch F, Panzer S, Marosi C, Birner P, Thaler J, Brostjan C, Lötsch D, Berger W, Hainfellner JA, Pabinger I, Ay C. Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood 2017; 129: 1831–9. - PMC - PubMed
-
- Suzuki‐Inoue K, Fuller GLJ, García A, Eble JA, Pöhlmann S, Inoue O, Gartner TK, Hughan SC, Pearce AC, Laing GD, Theakston RDG, Schweighoffer E, Zitzmann N, Morita T, Tybulewicz VLJ, Ozaki Y, Watson SP. A novel Syk‐dependent mechanism of platelet activation by the C‐type lectin receptor CLEC‐2. Blood 2006; 107: 542–9. - PubMed
-
- Shirai T, Inoue O, Tamura S, Tsukiji N, Sasaki T, Endo H, Satoh K, Osada M, Sato‐Uchida H, Fujii H, Ozaki Y, Suzuki‐Inoue K. C‐type lectin‐like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor‐bearing mice. J Thromb Haemost JTH 2017; 15: 513–25. - PubMed
-
- Hitchcock JR, Cook CN, Bobat S, Ross EA, Flores‐Langarica A, Lowe KL, Khan M, Dominguez‐Medina CC, Lax S, Carvalho‐Gaspar M, Hubscher S, Rainger GE, Cobbold M, Buckley CD, Mitchell TJ, Mitchell A, Jones ND, Van Rooijen N, Kirchhofer D, Henderson IR, et al Inflammation drives thrombosis after Salmonella infection via CLEC‐2 on platelets. J Clin Invest 2015; 125: 4429–46. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

