Endoplasmic reticulum stress-mediated autophagy protects against β,β-dimethylacrylshikonin-induced apoptosis in lung adenocarcinoma cells
- PMID: 29676829
- PMCID: PMC5989738
- DOI: 10.1111/cas.13616
Endoplasmic reticulum stress-mediated autophagy protects against β,β-dimethylacrylshikonin-induced apoptosis in lung adenocarcinoma cells
Abstract
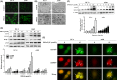
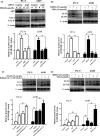
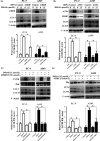
β,β-Dimethylacrylshikonin (DMAS) is an anti-cancer compound extracted from the roots of Lithospermum erythrorhizon. The present study aims to investigate the effects of DMAS on human lung adenocarcinoma cells in vitro and explore the mechanisms of its anti-cancer action. We showed that DMAS markedly inhibited cell viability in a dose- and time-dependent way, and induced apoptosis as well as autophagy in human lung adenocarcinoma cells. Furthermore, we found that DMAS stimulated endoplasmic reticulum stress and mediated autophagy through the PERK-eIF2α-ATF4-CHOP and IRE1-TRAF2-JNK axes of the unfolded protein response in human lung adenocarcinoma cells. We also showed that the autophagy induced by DMAS played a prosurvival role in human lung adenocarcinoma cells and attenuated the apoptotic cascade. Collectively, combined treatment of DMAS and pharmacological autophagy inhibitors could offer an effective therapeutic strategy for lung adenocarcinoma treatment.
Keywords: apoptosis; autophagy; endoplasmic reticulum stress; lung adenocarcinoma; β,β-dimethylacrylshikonin.
© 2018 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures




Similar articles
-
Activation of CaMKKβ-AMPK-mTOR pathway is required for autophagy induction by β,β-dimethylacrylshikonin against lung adenocarcinoma cells.Biochem Biophys Res Commun. 2019 Sep 24;517(3):477-483. doi: 10.1016/j.bbrc.2019.07.100. Epub 2019 Jul 31. Biochem Biophys Res Commun. 2019. PMID: 31376944
-
β, β-Dimethylacrylshikonin induces mitochondria-dependent apoptosis of human lung adenocarcinoma cells in vitro via p38 pathway activation.Acta Pharmacol Sin. 2015 Jan;36(1):131-8. doi: 10.1038/aps.2014.108. Epub 2014 Dec 1. Acta Pharmacol Sin. 2015. PMID: 25434989 Free PMC article.
-
Salinomycin induces cell death with autophagy through activation of endoplasmic reticulum stress in human cancer cells.Autophagy. 2013 Jul;9(7):1057-68. doi: 10.4161/auto.24632. Epub 2013 Apr 18. Autophagy. 2013. PMID: 23670030 Free PMC article.
-
The Role of the PERK/eIF2α/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress.Curr Mol Med. 2016;16(6):533-44. doi: 10.2174/1566524016666160523143937. Curr Mol Med. 2016. PMID: 27211800 Free PMC article. Review.
-
Inhibition of autophagy enhances heat-induced apoptosis in human non-small cell lung cancer cells through ER stress pathways.Arch Biochem Biophys. 2016 Oct 1;607:55-66. doi: 10.1016/j.abb.2016.08.016. Epub 2016 Aug 24. Arch Biochem Biophys. 2016. PMID: 27565443 Review.
Cited by
-
Autophagy Plays a Critical Role in Insulin Resistance- Mediated Chemoresistance in Hepatocellular Carcinoma Cells by Regulating the ER Stress.J Cancer. 2018 Oct 21;9(23):4314-4324. doi: 10.7150/jca.27943. eCollection 2018. J Cancer. 2018. PMID: 30519335 Free PMC article.
-
Sea Cucumber Derived Triterpenoid Glycoside Frondoside A: A Potential Anti-Bladder Cancer Drug.Nutrients. 2023 Jan 11;15(2):378. doi: 10.3390/nu15020378. Nutrients. 2023. PMID: 36678249 Free PMC article.
-
The pathogenesis and potential therapeutic targets in sepsis.MedComm (2020). 2023 Nov 20;4(6):e418. doi: 10.1002/mco2.418. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38020710 Free PMC article. Review.
-
Development of a pH-responsive polymersome inducing endoplasmic reticulum stress and autophagy blockade.Sci Adv. 2020 Jul 31;6(31):eabb8725. doi: 10.1126/sciadv.abb8725. eCollection 2020 Jul. Sci Adv. 2020. PMID: 32789182 Free PMC article.
-
Identification of endoplasmic reticulum stress-related lncRNAs in lung adenocarcinoma by bioinformatics and experimental validation.Ann Med. 2023;55(2):2251500. doi: 10.1080/07853890.2023.2251500. Ann Med. 2023. PMID: 37643369 Free PMC article.
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. - PubMed
-
- Hanagiri T, Baba T, So T, et al. Time trends of surgical outcome of patients with non‐small cell lung cancer. J Thorac Oncol. 2010;5:825‐829. - PubMed
-
- Langer CJ, Albert I, Ross HJ, et al. Randomized phase II study of carboplatin and etoposide with or without obatoclax mesylate in extensive‐stage small cell lung cancer. Lung Cancer. 2014;85:420‐428. - PubMed
-
- Frederiks CN, Lam SW, Guchelaar HJ, Boven E. Genetic polymorphisms and paclitaxel‐ or docetaxel‐induced toxicities: a systematic review. Cancer Treat Rev. 2015;41:930‐950. - PubMed
-
- Niemeijer ND, Alblas G, van Hulsteijn LT, Dekkers OM, Corssmit EP. Chemotherapy with cyclophosphamide, vincristine and dacarbazine for malignant paraganglioma and pheochromocytoma: systematic review and meta‐analysis. Clin Endocrinol (Oxf). 2014;81:642‐651. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

