β-Glucocerebrosidase Modulators Promote Dimerization of β-Glucocerebrosidase and Reveal an Allosteric Binding Site
- PMID: 29676907
- PMCID: PMC6098685
- DOI: 10.1021/jacs.7b13003
β-Glucocerebrosidase Modulators Promote Dimerization of β-Glucocerebrosidase and Reveal an Allosteric Binding Site
Abstract
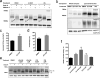
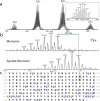
β-Glucocerebrosidase (GCase) mutations cause Gaucher's disease and are a high risk factor in Parkinson's disease. The implementation of a small molecule modulator is a strategy to restore proper folding and lysosome delivery of degradation-prone mutant GCase. Here, we present a potent quinazoline modulator, JZ-4109, which stabilizes wild-type and N370S mutant GCase and increases GCase abundance in patient-derived fibroblast cells. We then developed a covalent modification strategy using a lysine targeted inactivator (JZ-5029) for in vitro mechanistic studies. By using native top-down mass spectrometry, we located two potentially covalently modified lysines. We obtained the first crystal structure, at 2.2 Å resolution, of a GCase with a noniminosugar modulator covalently bound, and were able to identify the exact lysine residue modified (Lys346) and reveal an allosteric binding site. GCase dimerization was induced by our modulator binding, which was observed by native mass spectrometry, its crystal structure, and size exclusion chromatography with a multiangle light scattering detector. Finally, the dimer form was confirmed by negative staining transmission electron microscopy studies. Our newly discovered allosteric site and observed GCase dimerization provide a new mechanistic insight into GCase and its noniminosugar modulators and facilitate the rational design of novel GCase modulators for Gaucher's disease and Parkinson's disease.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

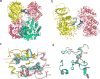



Similar articles
-
Novel β-Glucocerebrosidase Activators That Bind to a New Pocket at a Dimer Interface and Induce Dimerization.Angew Chem Int Ed Engl. 2021 Mar 1;60(10):5436-5442. doi: 10.1002/anie.202013890. Epub 2021 Jan 19. Angew Chem Int Ed Engl. 2021. PMID: 33238058
-
Design and Synthesis of Potent Quinazolines as Selective β-Glucocerebrosidase Modulators.J Med Chem. 2016 Sep 22;59(18):8508-20. doi: 10.1021/acs.jmedchem.6b00930. Epub 2016 Sep 6. J Med Chem. 2016. PMID: 27598312 Free PMC article.
-
Fragment-Based Discovery of a Series of Allosteric-Binding Site Modulators of β-Glucocerebrosidase.J Med Chem. 2024 Jul 11;67(13):11168-11181. doi: 10.1021/acs.jmedchem.4c00702. Epub 2024 Jun 27. J Med Chem. 2024. PMID: 38932616
-
The interplay between Glucocerebrosidase, α-synuclein and lipids in human models of Parkinson's disease.Biophys Chem. 2021 Jun;273:106534. doi: 10.1016/j.bpc.2020.106534. Epub 2020 Dec 25. Biophys Chem. 2021. PMID: 33832803 Review.
-
Klotho-Related Protein KLrP: Structure and Functions.Vitam Horm. 2016;101:1-16. doi: 10.1016/bs.vh.2016.02.011. Epub 2016 Mar 29. Vitam Horm. 2016. PMID: 27125736 Review.
Cited by
-
Development of selective nanomolar cyclic peptide ligands as GBA1 enzyme stabilisers.RSC Chem Biol. 2025 Jan 31;6(4):563-570. doi: 10.1039/d4cb00218k. eCollection 2025 Apr 2. RSC Chem Biol. 2025. PMID: 39936129 Free PMC article.
-
A modulator of wild-type glucocerebrosidase improves pathogenic phenotypes in dopaminergic neuronal models of Parkinson's disease.Sci Transl Med. 2019 Oct 16;11(514):eaau6870. doi: 10.1126/scitranslmed.aau6870. Sci Transl Med. 2019. PMID: 31619543 Free PMC article.
-
GCase Enhancers: A Potential Therapeutic Option for Gaucher Disease and Other Neurological Disorders.Pharmaceuticals (Basel). 2022 Jul 2;15(7):823. doi: 10.3390/ph15070823. Pharmaceuticals (Basel). 2022. PMID: 35890122 Free PMC article. Review.
-
Evaluation of Strategies for Measuring Lysosomal Glucocerebrosidase Activity.Mov Disord. 2021 Dec;36(12):2719-2730. doi: 10.1002/mds.28815. Epub 2021 Oct 6. Mov Disord. 2021. PMID: 34613624 Free PMC article. Review.
-
Activation and Purification of ß-Glucocerebrosidase by Exploiting its Transporter LIMP-2 - Implications for Novel Treatment Strategies in Gaucher's and Parkinson's Disease.Adv Sci (Weinh). 2024 Jul;11(25):e2401641. doi: 10.1002/advs.202401641. Epub 2024 Apr 26. Adv Sci (Weinh). 2024. PMID: 38666485 Free PMC article.
References
-
- Grabowski GA. Lancet. 2008;372:1263. - PubMed
-
- Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. N. Engl. J. Med. 2004;351:1972. - PubMed
-
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Durr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG. N. Engl. J. Med. 2009;361:1651. - PMC - PubMed
-
- Schapira AHV, Olanow CW, Greenamyre JT, Bezard E. Lancet. 2014;384:545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

