β-Glucocerebrosidase Modulators Promote Dimerization of β-Glucocerebrosidase and Reveal an Allosteric Binding Site
- PMID: 29676907
- PMCID: PMC6098685
- DOI: 10.1021/jacs.7b13003
β-Glucocerebrosidase Modulators Promote Dimerization of β-Glucocerebrosidase and Reveal an Allosteric Binding Site
Abstract
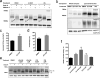
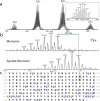
β-Glucocerebrosidase (GCase) mutations cause Gaucher's disease and are a high risk factor in Parkinson's disease. The implementation of a small molecule modulator is a strategy to restore proper folding and lysosome delivery of degradation-prone mutant GCase. Here, we present a potent quinazoline modulator, JZ-4109, which stabilizes wild-type and N370S mutant GCase and increases GCase abundance in patient-derived fibroblast cells. We then developed a covalent modification strategy using a lysine targeted inactivator (JZ-5029) for in vitro mechanistic studies. By using native top-down mass spectrometry, we located two potentially covalently modified lysines. We obtained the first crystal structure, at 2.2 Å resolution, of a GCase with a noniminosugar modulator covalently bound, and were able to identify the exact lysine residue modified (Lys346) and reveal an allosteric binding site. GCase dimerization was induced by our modulator binding, which was observed by native mass spectrometry, its crystal structure, and size exclusion chromatography with a multiangle light scattering detector. Finally, the dimer form was confirmed by negative staining transmission electron microscopy studies. Our newly discovered allosteric site and observed GCase dimerization provide a new mechanistic insight into GCase and its noniminosugar modulators and facilitate the rational design of novel GCase modulators for Gaucher's disease and Parkinson's disease.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

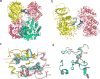



References
-
- Grabowski GA. Lancet. 2008;372:1263. - PubMed
-
- Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. N. Engl. J. Med. 2004;351:1972. - PubMed
-
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Durr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG. N. Engl. J. Med. 2009;361:1651. - PMC - PubMed
-
- Schapira AHV, Olanow CW, Greenamyre JT, Bezard E. Lancet. 2014;384:545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

