Ocular Delivery System for Propranolol Hydrochloride Based on Nanostructured Lipid Carrier
- PMID: 29677103
- PMCID: PMC6027676
- DOI: 10.3390/scipharm86020016
Ocular Delivery System for Propranolol Hydrochloride Based on Nanostructured Lipid Carrier
Abstract
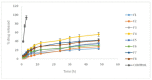
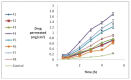
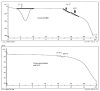
One drawback of traditional forms of medical ocular dosage is drug dilution by tear; moreover, drugs are rapidly drained away from pre-corneal cavity by tear flow and lacrimo-nasal drainage. Prolonging contact time with different strategies and mucoadhesive vehicles will help to continuously deliver drugs to the eyes. For this study, we prepared and evaluated the effects of a nanostructure lipid carrier (NLC) on propranolol hydrochloride as a hydrophilic drug model for rabbit corneal permeation. Propranolol hydrochloride NLC was prepared using cold homogenization. The lipid was melted, then the drug and surfactant were dispersed and stirred into the melted lipid. This fused lipid phase was scattered in aqueous solution containing the cosurfactant at 4 °C and then homogenized. We evaluated particle size, drug loading, drug release, and NLC permeability through rabbit cornea as well as the formula’s effect on the cornea. Our results show that drug loading efficiency depended on the surfactant/lipid ratio (S/L) and the percentages of liquid lipid and Transcutol (Gattefosse, Saint-Priest, France) (as solubilizer). Drug release data were evaluated with the Higuchi model and a significant correlation was shown between the S/L ratio and the amount of drug released after 4 and 48 h. NLC formulations improved propranolol hydrochloride permeation. We conclude that the effect of the NLC formulations was due to mucoadhesive and film forming properties.
Keywords: drug delivery; nanostructured lipid carrier; ocular; permeability; propranolol hydrochloride.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Transport mechanism of chitosan-N-acetylcysteine, chitosan oligosaccharides or carboxymethyl chitosan decorated coumarin-6 loaded nanostructured lipid carriers across the rabbit ocular.Eur J Pharm Biopharm. 2017 Nov;120:89-97. doi: 10.1016/j.ejpb.2017.08.013. Epub 2017 Sep 1. Eur J Pharm Biopharm. 2017. PMID: 28867370
-
Phenylboronic acid-tethered chondroitin sulfate-based mucoadhesive nanostructured lipid carriers for the treatment of dry eye syndrome.Acta Biomater. 2019 Nov;99:350-362. doi: 10.1016/j.actbio.2019.08.035. Epub 2019 Aug 23. Acta Biomater. 2019. PMID: 31449929
-
Surface engineered nanostructured lipid carriers for efficient nose to brain delivery of ondansetron HCl using Delonix regia gum as a natural mucoadhesive polymer.Colloids Surf B Biointerfaces. 2014 Oct 1;122:143-150. doi: 10.1016/j.colsurfb.2014.06.037. Epub 2014 Jul 2. Colloids Surf B Biointerfaces. 2014. PMID: 25033434
-
[Preparation of isopsoralen loaded nanostructured carrier and its in vitro transdermal permeation characteristics].Zhongguo Zhong Yao Za Zhi. 2017 Jul;42(13):2473-2478. doi: 10.19540/j.cnki.cjcmm.20170507.001. Zhongguo Zhong Yao Za Zhi. 2017. PMID: 28840686 Chinese.
-
Design and evaluations of a nanostructured lipid carrier loaded with dopamine hydrochloride for intranasal bypass drug delivery in Parkinson's disease.J Microencapsul. 2023 Dec;40(8):599-612. doi: 10.1080/02652048.2023.2264386. Epub 2023 Nov 9. J Microencapsul. 2023. PMID: 37787159
Cited by
-
Nanostructured Lipid Carriers Mediated Drug Delivery to Posterior Segment of Eye and their In-vivo Successes.Curr Pharm Biotechnol. 2024;25(6):713-723. doi: 10.2174/1389201025666230907145019. Curr Pharm Biotechnol. 2024. PMID: 37691214 Review.
-
Hypolipidemic Activity of Olive Oil-Based Nanostructured Lipid Carrier Containing Atorvastatin.Nanomaterials (Basel). 2022 Jun 23;12(13):2160. doi: 10.3390/nano12132160. Nanomaterials (Basel). 2022. PMID: 35807995 Free PMC article.
-
Nanostructured Lipid Carrier-loaded In Situ Gel for Ophthalmic Drug Delivery: Preparation and In Vitro Characterization Studies.Pharm Nanotechnol. 2025;13(1):171-183. doi: 10.2174/0122117385266639231029192409. Pharm Nanotechnol. 2025. PMID: 38213174
-
Cannabidiol-Loaded Nanostructured Lipid Carriers (NLCs) for Dermal Delivery: Enhancement of Photostability, Cell Viability, and Anti-Inflammatory Activity.Pharmaceutics. 2023 Feb 6;15(2):537. doi: 10.3390/pharmaceutics15020537. Pharmaceutics. 2023. PMID: 36839859 Free PMC article.
-
Lipid nanostructures for targeting brain cancer.Heliyon. 2021 Sep 16;7(9):e07994. doi: 10.1016/j.heliyon.2021.e07994. eCollection 2021 Sep. Heliyon. 2021. PMID: 34632135 Free PMC article. Review.
References
-
- Mitra A.K. Ophthalmic Drug Delivery Systems. Volume 130. Taylor & Francis Inc; New York, NY USA: 2003. pp. 178–197.
-
- Macha S., Hughes P., Mitra A. Drugs and the Pharmaceutical Sciences. Volume 130. CRC Press; Boca Raton, FL, USA: 2003. Overview of ocular drug delivery; pp. 1–12.
-
- Sánchez-López E., Espina M., Doktorovova S., Souto E., García M. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye–part i–barriers and determining factors in ocular delivery. Eur. J. Pharm. Biopharm. 2017;110:70–75. doi: 10.1016/j.ejpb.2016.10.009. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
