SmSP2: A serine protease secreted by the blood fluke pathogen Schistosoma mansoni with anti-hemostatic properties
- PMID: 29677188
- PMCID: PMC5931690
- DOI: 10.1371/journal.pntd.0006446
SmSP2: A serine protease secreted by the blood fluke pathogen Schistosoma mansoni with anti-hemostatic properties
Abstract
Background: Serine proteases are important virulence factors for many pathogens. Recently, we discovered a group of trypsin-like serine proteases with domain organization unique to flatworm parasites and containing a thrombospondin type 1 repeat (TSR-1). These proteases are recognized as antigens during host infection and may prove useful as anthelminthic vaccines, however their molecular characteristics are under-studied. Here, we characterize the structural and proteolytic attributes of serine protease 2 (SmSP2) from Schistosoma mansoni, one of the major species responsible for the tropical infectious disease, schistosomiasis.
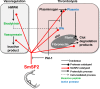
Methodology/principal findings: SmSP2 comprises three domains: a histidine stretch, TSR-1 and a serine protease domain. The cleavage specificity of recombinant SmSP2 was determined using positional scanning and multiplex combinatorial libraries and the determinants of specificity were identified with 3D homology models, demonstrating a trypsin-like endopeptidase mode of action. SmSP2 displayed restricted proteolysis on protein substrates. It activated tissue plasminogen activator and plasminogen as key components of the fibrinolytic system, and released the vasoregulatory peptide, kinin, from kininogen. SmSP2 was detected in the surface tegument, esophageal glands and reproductive organs of the adult parasite by immunofluorescence microscopy, and in the excretory/secretory products by immunoblotting.
Conclusions/significance: The data suggest that SmSP2 is secreted, functions at the host-parasite interface and contributes to the survival of the parasite by manipulating host vasodilatation and fibrinolysis. SmSP2 may be, therefore, a potential target for anti-schistosomal therapy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6(7):411–25. S1473-3099(06)70521-7 [pii]; doi: 10.1016/S1473-3099(06)70521-7 - DOI - PubMed
-
- King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113(2):95–104. doi: 10.1016/j.actatropica.2009.11.012 ; PubMed Central PMCID: PMCPMC2812649. - DOI - PMC - PubMed
-
- Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368(9541):1106–18. doi: 10.1016/S0140-6736(06)69440-3 - DOI - PubMed
-
- Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, McManus DP. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009;31(4):163–76. PIM1098 [pii]; doi: 10.1111/j.1365-3024.2009.01098.x - DOI - PubMed
-
- Caffrey CR. Chemotherapy of schistosomiasis: present and future. Curr Opin Chem Biol. 2007;11(4):433–9. doi: 10.1016/j.cbpa.2007.05.031 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

