Osteopontin plays a pivotal role in increasing severity of respiratory syncytial virus infection
- PMID: 29677209
- PMCID: PMC5909912
- DOI: 10.1371/journal.pone.0192709
Osteopontin plays a pivotal role in increasing severity of respiratory syncytial virus infection
Abstract
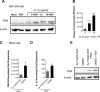
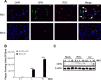
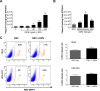
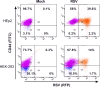
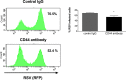
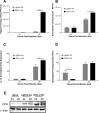
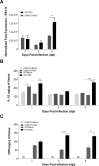
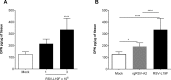
The molecular mechanisms underlying susceptibility to severe respiratory syncytial virus (RSV) infection remain poorly understood. Herein, we report on the role of osteopontin (OPN) in regulation of RSV infection in human epithelial cells and how interleukin-1 beta (IL-1β), a cytokine secreted soon after RSV infection, when persistently expressed can induce OPN expression leading to increased viral infection. We first compared OPN expression in two human epithelial cell lines: HEK-293 and HEp-2. In contrast to HEp-2, HEK-293 expresses low levels of pro-caspase-1 resulting in decreased IL-1β expression in response to RSV infection. We found a correlation between low IL-1β levels and a delay in induction of OPN expression in RSV-infected HEK-293 cells compared to HEp-2. This phenomenon could partially explain the high susceptibility of HEp-2 cells to RSV infection versus the moderate susceptibility of HEK-293 cells. Also, HEK-293 cells expressing low levels of pro-caspase-1 exhibit decreased IL-1β expression and delayed OPN expression in response to RSV infection. HEK-293 cells incubated with human rIL-1β showed a dose-dependent increase in OPN expression upon RSV infection. Also, incubation with rOPN increased RSV viral load. Moreover, HEp-2 cells or mice infected with a mucogenic RSV strain RSV-L19F showed elevated levels of OPN in contrast to mice infected with the laboratory RSV strain rA2. This correlated with elevated levels of OPN following infection with RSV-L19F compared to rA2. Together, these results demonstrate that increased OPN expression is regulated in part by IL-1β, and the interplay between IL-1β and OPN signaling may play a pivotal role in the spread of RSV infection.
Conflict of interest statement
Figures
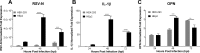

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

