Association Between Baseline LDL-C Level and Total and Cardiovascular Mortality After LDL-C Lowering: A Systematic Review and Meta-analysis
- PMID: 29677301
- PMCID: PMC5933331
- DOI: 10.1001/jama.2018.2525
Association Between Baseline LDL-C Level and Total and Cardiovascular Mortality After LDL-C Lowering: A Systematic Review and Meta-analysis
Erratum in
-
Incorrect Data or Information.JAMA. 2018 Oct 2;320(13):1387. doi: 10.1001/jama.2018.12240. JAMA. 2018. PMID: 30285158 Free PMC article. No abstract available.
Abstract
Importance: Effects on specific fatal and nonfatal end points appear to vary for low-density lipoprotein cholesterol (LDL-C)-lowering drug trials.
Objective: To evaluate whether baseline LDL-C level is associated with total and cardiovascular mortality risk reductions.
Data sourcesand study selection: Electronic databases (Cochrane, MEDLINE, EMBASE, TCTMD, ClinicalTrials.gov, major congress proceedings) were searched through February 2, 2018, to identify randomized clinical trials of statins, ezetimibe, and PCSK9-inhibiting monoclonal antibodies.
Data extraction and synthesis: Two investigators abstracted data and appraised risks of bias. Intervention groups were categorized as "more intensive" (more potent pharmacologic intervention) or "less intensive" (less potent, placebo, or control group).
Main outcomes and measures: The coprimary end points were total mortality and cardiovascular mortality. Random-effects meta-regression and meta-analyses evaluated associations between baseline LDL-C level and reductions in mortality end points and secondary end points including major adverse cardiac events (MACE).
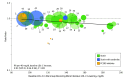
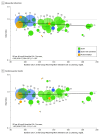
Results: In 34 trials, 136 299 patients received more intensive and 133 989 received less intensive LDL-C lowering. All-cause mortality was lower for more vs less intensive therapy (7.08% vs 7.70%; rate ratio [RR], 0.92 [95% CI, 0.88 to 0.96]), but varied by baseline LDL-C level. Meta-regression showed more intensive LDL-C lowering was associated with greater reductions in all-cause mortality with higher baseline LDL-C levels (change in RRs per 40-mg/dL increase in baseline LDL-C, 0.91 [95% CI, 0.86 to 0.96]; P = .001; absolute risk difference [ARD], -1.05 incident cases per 1000 person-years [95% CI, -1.59 to -0.51]), but only when baseline LDL-C levels were 100 mg/dL or greater (P < .001 for interaction) in a meta-analysis. Cardiovascular mortality was lower for more vs less intensive therapy (3.48% vs 4.07%; RR, 0.84 [95% CI, 0.79 to 0.89]) but varied by baseline LDL-C level. Meta-regression showed more intensive LDL-C lowering was associated with a greater reduction in cardiovascular mortality with higher baseline LDL-C levels (change in RRs per 40-mg/dL increase in baseline LDL-C, 0.86 [95% CI, 0.80 to 0.94]; P < .001; ARD, -1.0 incident cases per 1000 person-years [95% CI, -1.51 to -0.45]), but only when baseline LDL-C levels were 100 mg/dL or greater (P < .001 for interaction) in a meta-analysis. Trials with baseline LDL-C levels of 160 mg/dL or greater had the greatest reduction in all-cause mortality (RR, 0.72 [95% CI, 0.62 to 0.84]; P < .001; 4.3 fewer deaths per 1000 person-years) in a meta-analysis. More intensive LDL-C lowering was also associated with progressively greater risk reductions with higher baseline LDL-C level for myocardial infarction, revascularization, and MACE.
Conclusions and relevance: In these meta-analyses and meta-regressions, more intensive compared with less intensive LDL-C lowering was associated with a greater reduction in risk of total and cardiovascular mortality in trials of patients with higher baseline LDL-C levels. This association was not present when baseline LDL-C level was less than 100 mg/dL, suggesting that the greatest benefit from LDL-C-lowering therapy may occur for patients with higher baseline LDL-C levels.
Conflict of interest statement
Figures


Comment in
-
Challenges in Interpreting the Lipid-Lowering Trials: Biology vs Ecology.JAMA. 2018 Apr 17;319(15):1549-1551. doi: 10.1001/jama.2018.4041. JAMA. 2018. PMID: 29677285 No abstract available.
-
Review: More-intensive vs less-intensive LDL-cholesterol lowering reduces mortality.Ann Intern Med. 2018 Jul 17;169(2):JC6. doi: 10.7326/ACPJC-2018-169-2-006. Ann Intern Med. 2018. PMID: 30014095 No abstract available.
-
Meta-analysis of LDL-C Lowering and Mortality.JAMA. 2018 Oct 9;320(14):1493. doi: 10.1001/jama.2018.11202. JAMA. 2018. PMID: 30304421 No abstract available.
References
-
- Cannon CP, Blazing MA, Giugliano RP, et al. ; IMPROVE-IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. - PubMed
-
- Sabatine MS, Giugliano RP, Keech AC, et al. ; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. - PubMed
-
- Robinson JG, Farnier M, Krempf M, et al. ; ODYSSEY LONG TERM Investigators . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489-1499. - PubMed
-
- Sabatine MS, Giugliano RP, Wiviott SD, et al. ; Open-Label Study of Long-Term Evaluation Against LDL Cholesterol (OSLER) Investigators . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500-1509. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

