Complement and the Regulation of T Cell Responses
- PMID: 29677470
- PMCID: PMC7478175
- DOI: 10.1146/annurev-immunol-042617-053245
Complement and the Regulation of T Cell Responses
Abstract
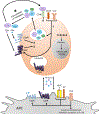
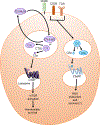
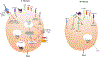
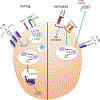
The complement system is an evolutionarily ancient key component of innate immunity required for the detection and removal of invading pathogens. It was discovered more than 100 years ago and was originally defined as a liver-derived, blood-circulating sentinel system that classically mediates the opsonization and lytic killing of dangerous microbes and the initiation of the general inflammatory reaction. More recently, complement has also emerged as a critical player in adaptive immunity via its ability to instruct both B and T cell responses. In particular, work on the impact of complement on T cell responses led to the surprising discoveries that the complement system also functions within cells and is involved in regulating basic cellular processes, predominantly those of metabolic nature. Here, we review current knowledge about complement's role in T cell biology, with a focus on the novel intracellular and noncanonical activities of this ancient system.
Keywords: CD46; Th1 response; autoimmunity; complosome; infection; metabolism.
Figures
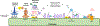

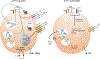

References
-
- Creagh EM, O’Neill LA. 2006. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 27:352–57 - PubMed
-
- Köhl J 2006. The role of complement in danger sensing and transmission. Immunol. Res. 34:157–76 - PubMed
-
- Walport M 2001. Complement. First of two parts. N. Engl. J. Med. 344:1058–66 - PubMed
-
- Walport M 2001. Complement. Second of two parts. N. Engl. J. Med. 344:1140–44 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

