Evaluation of PET and laparoscopy in STagIng advanced gastric cancer: a multicenter prospective study (PLASTIC-study)
- PMID: 29678145
- PMCID: PMC5910577
- DOI: 10.1186/s12885-018-4367-9
Evaluation of PET and laparoscopy in STagIng advanced gastric cancer: a multicenter prospective study (PLASTIC-study)
Abstract
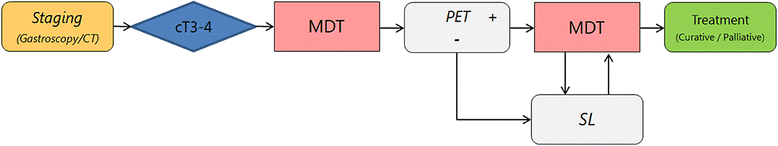
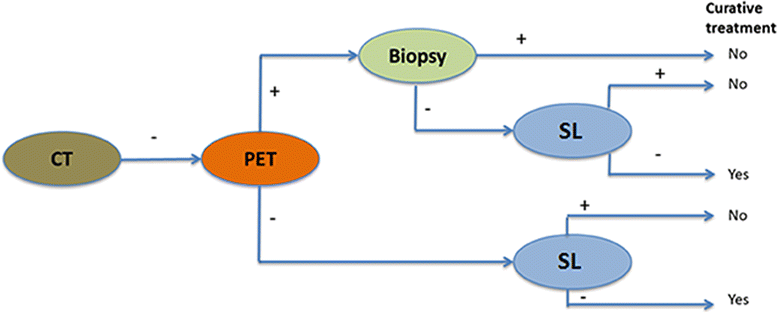
Background: Initial staging of gastric cancer consists of computed tomography (CT) and gastroscopy. In locally advanced (cT3-4) gastric cancer, fluorodeoxyglucose positron emission tomography with CT (FDG-PET/CT or PET) and staging laparoscopy (SL) may have a role in staging, but evidence is scarce. The aim of this study is to evaluate the impact and cost-effectiveness of PET and SL in addition to initial staging in patients with locally advanced gastric cancer.
Methods: This prospective observational cohort study will include all patients with a surgically resectable, advanced gastric adenocarcinoma (cT3-4b, N0-3, M0), that are scheduled for treatment with curative intent after initial staging with gastroscopy and CT. The modalities to be investigated in this study is the addition of PET and SL. The primary outcome of this study is the proportion of patients in whom the PET or SL lead to a change in treatment strategy. Secondary outcome parameters are: diagnostic performance, morbidity and mortality, quality of life, and cost-effectiveness of these additional diagnostic modalities. The study recently started in August 2017 with a duration of 36 months. At least 239 patients need to be included in this study to demonstrate that the diagnostic modalities are break-even. Based on the annual number of gastrectomies in the participating centers, it is estimated that approximately 543 patients are included in this study.
Discussion: In this study, it is hypothesized that performing PET and SL for locally advanced gastric adenocarcinomas results in a change of treatment strategy in 27% of patients and an annual cost-reduction in the Netherlands of €916.438 in this patient group by reducing futile treatment. The results of this study may be applicable to all countries with comparable treatment algorithms and health care systems.
Trial registration: NCT03208621 . This trial was registered prospectively on June 30, 2017.
Keywords: Gastrectomy; Gastric cancer; Laparoscopy.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the ethical committee (METC, reference number: 16–633/C). As this study does not allocate patients to study interventions other than usual care, as recommended by the new Dutch guidelines, this study does not fall within the Medical Research Involving Human Subjects Act (WMO). A waiver for written informed consent was not obtained. Written informed consent will be obtained from all study participants.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial P Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. - DOI - PubMed
-
- Al-Batran S, Homann N, Schmalenberg H, Kopp H, Haag GM, Luley KB, Schmiegel WH, Folprecht G, Probst S, Prasnikar N, Thuss-Patience P, Fischbach W, Trojan J, Koenigsmann M, Pauligk C, Goetze TO, Jaeger E, Meiler J, Schuler MH, Hofheinz R. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): a multicenter, randomized phase 3 trial. JCO. 2017;35(15):4004.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

