Survival after recurrence in patients with gastric cancer who receive S-1 adjuvant chemotherapy: exploratory analysis of the ACTS-GC trial
- PMID: 29678146
- PMCID: PMC5910584
- DOI: 10.1186/s12885-018-4341-6
Survival after recurrence in patients with gastric cancer who receive S-1 adjuvant chemotherapy: exploratory analysis of the ACTS-GC trial
Abstract
Background: Some patients develop recurrence after curative resection and adjuvant chemotherapy. S-1, an oral fluoropyrimidine, is one of the standard regimens in adjuvant chemotherapy, and is also used in first-line treatment for advanced/metastatic gastric cancer. It is controversial as to whether the same treatment strategy can be applied for patients who develop recurrence after adjuvant chemotherapy and those who did not receive adjuvant chemotherapy. To investigate this issue, we compared the outcomes of patients who developed recurrences after treatment with or without adjuvant chemotherapy using the results of the Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer (ACTS-GC).
Methods: Patients who had confirmed recurrence in the ACTS-GC trial were analyzed. We defined 2 independent cohorts. Cohort 1 patients were divided by whether they received adjuvant chemotherapy (adjuvant S-1 group and surgery-only group). Cohort 2 patients were divided by whether they received a regimen including S-1 (IS) or not including S-1 (NIS) after recurrence.
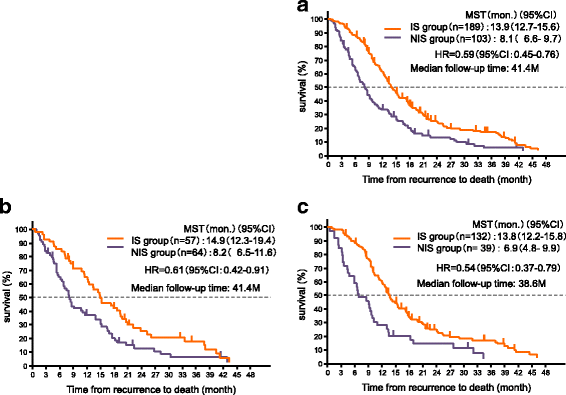
Results: A total of 375 patients experienced recurrence (160 in the adjuvant S-1 group and 215 in the surgery-only group). In cohort 1, the median time from recurrence to death (TFRD) was 11.4 months (95% confidence interval [CI], 8.4-13.9) in the adjuvant S-1 group and 11.3 months (95% CI, 9.7-13.1) in the surgery-only group (hazard ratio [HR], 1.05; 95% CI, 0.84-1.31). In cohort 2, 292 patients received chemotherapy after recurrence and were divided into the IS (n = 189) or the NIS group (n = 103). The median TFRD was 13.9 months (95% CI, 12.7-15.6) in the IS group and 8.1 months (95% CI, 6.6-9.7) in the NIS group (HR, 0.59; 95% CI, 0.45-0.76), and there was no significant interaction between the adjuvant S-1 group and surgery-only group.
Conclusion: Adjuvant chemotherapy with S-1 prolonged overall survival without influencing the TFRD. The same treatment strategy may be applied for patients who develop recurrence after adjuvant chemotherapy and those who did not receive adjuvant chemotherapy.
Trial registration: NCT00152217 . First Posted on September 9, 2005.
Keywords: ACTS-GC; Adjuvant chemotherapy; Recurrent gastric cancer; S-1; Stomach neoplasms.
Conflict of interest statement
Ethics approval and consent to participate
The ACTS-GC trial was conducted in accordance with the World Medical Association Declaration of Helsinki and Japanese Good Clinical Practice guidelines. The protocol of ACTS-GC was approved by the institutional review board of each participating hospital. The institutions participated in the ACTS-GC were shown in Additional file 1: Text S1. Written informed consent was obtained from all patients. Since this analysis was an ad-hoc exploratory analysis, we did not seek additional approval and consent.
Competing interests
SI received honoraria from Taiho, Chugai, Yakult Honsha, Covidien, Eli Lilly, Takeda, Nippon Kayaku, Kyowa Hakko Kirin, and a grant from Chugai, Yakult Honsha, and writing assistance from Taiho; YO has stock in Statcom and received personal fees as executive salaries from Statcom, honoraria from Sanofi and Eisai, and consulting fees from Chugai, Taiho, Shionogi, Kowa and Eisai, and travel expenses from Yakult Honsha, and Takeda; MS received honoraria from Taiho, Yakult Honsha, Chugai, Eli Lilly, Ono, Takeda, and Sanofi, and grants from Taiho, Yakult Honsha, Eli Lilly, Sanofi, and Takeda.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Mari E, Floriani I, Tinazzi A, Buda A, Belfiglio M, Valentini M, et al. Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo studio dei Carcinomi dell'Apparato Digerente) Ann Oncol. 2000;11(7):837–843. doi: 10.1023/A:1008377101672. - DOI - PubMed
-
- Panzini I, Gianni L, Fattori PP, Tassinari D, Imola M, Fabbri P, et al. Adjuvant chemotherapy in gastric cancer: a meta-analysis of randomized trials and a comparison with previous meta-analyses. Tumori. 2002;88(1):21–27. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

