A20 deubiquitinase controls PGC-1α expression in the adipose tissue
- PMID: 29678181
- PMCID: PMC5909260
- DOI: 10.1186/s12944-018-0740-6
A20 deubiquitinase controls PGC-1α expression in the adipose tissue
Abstract
Background: Peroxisome proliferator-activated receptor γ coactivator- 1alpha (PGC-1α) plays an important role in whole body metabolism and, particularly in glucose homeostasis. Its expression is highly regulated and, small variations in tissue levels can have a major impact in a number of physiological and pathological conditions. Recent studies have shown that the ubiquitin/proteasome system plays a role in the control of PGC-1α degradation.
Methods: Here we evaluated the interaction of PGC-1α with the protein A20, which plays a dual-role in the control of the ubiquitin/proteasome system acting as a deubiquitinase and as an E3 ligase. We employed immunoprecipitation, quantitative real-time PCR and immunofluorescence staining to evaluate PGC-1α, A20, PPARγ and ubiquitin in the adipose tissue of humans and mice.
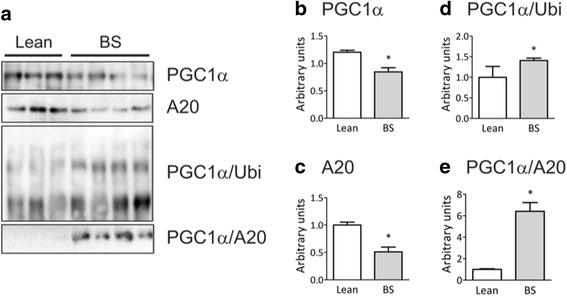
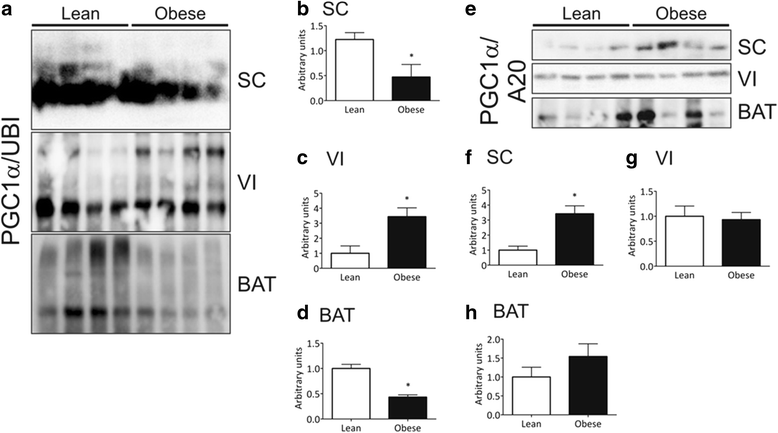
Results: In distinct sites of the adipose tissue, A20 binds to PGC-1α. At least in the subcutaneous fat of humans and mice the levels of PGC-1α decrease during obesity, while its physical association with A20 increases. The inhibition of A20 leads to a reduction of PGC-1α and PPARγ expression, suggesting that A20 acts as a protective factor against PGC-1α disposal.
Conclusion: We provide evidence that mechanisms regulating PGC-1α ubiquitination are potentially involved in the control of the function of this transcriptional co-activator.
Keywords: Diet; Fat; Glucose; Obesity; Ubiquitination.
Conflict of interest statement
Ethics approval and consent to participate
The human part of the study was approved by the University of Campinas Ethics Committee for Medical Research (#833/2010). The experimental part of the study were performed in accordance with the guidelines of the Brazilian College for Animal Experimentation and were approved by the University of Campinas Ethics Committee (#CEUA 2216–1).
Consent for publication
All authors agree with this publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Bargut TCL, Souza-Mello V, Aguila MB, Mandarim-de-Lacerda CA: Browning of white adipose tissue: lessons from experimental models. Horm Mol Biol Clin Investig 2017;31:1–13. - PubMed
-
- De Souza CT, Gasparetti AL, Pereira-da-Silva M, Araujo EP, Carvalheira JB, Saad MJ, Boschero AC, Carneiro EM, Velloso LA. Peroxisome proliferator-activated receptor gamma coactivator-1-dependent uncoupling protein-2 expression in pancreatic islets of rats: a novel pathway for neural control of insulin secretion. Diabetologia. 2003;46:1522–1531. doi: 10.1007/s00125-003-1222-5. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

