Growth hormone receptor-deficient pigs resemble the pathophysiology of human Laron syndrome and reveal altered activation of signaling cascades in the liver
- PMID: 29678421
- PMCID: PMC6001387
- DOI: 10.1016/j.molmet.2018.03.006
Growth hormone receptor-deficient pigs resemble the pathophysiology of human Laron syndrome and reveal altered activation of signaling cascades in the liver
Abstract
Objective: Laron syndrome (LS) is a rare, autosomal recessive disorder in humans caused by loss-of-function mutations of the growth hormone receptor (GHR) gene. To establish a large animal model for LS, pigs with GHR knockout (KO) mutations were generated and characterized.
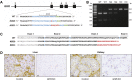
Methods: CRISPR/Cas9 technology was applied to mutate exon 3 of the GHR gene in porcine zygotes. Two heterozygous founder sows with a 1-bp or 7-bp insertion in GHR exon 3 were obtained, and their heterozygous F1 offspring were intercrossed to produce GHR-KO, heterozygous GHR mutant, and wild-type pigs. Since the latter two groups were not significantly different in any parameter investigated, they were pooled as the GHR expressing control group. The characterization program included body and organ growth, body composition, endocrine and clinical-chemical parameters, as well as signaling studies in liver tissue.
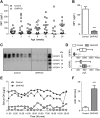
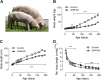
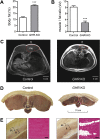
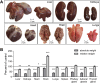
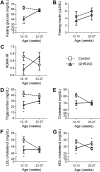
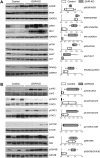
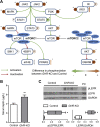
Results: GHR-KO pigs lacked GHR and had markedly reduced serum insulin-like growth factor 1 (IGF1) levels and reduced IGF-binding protein 3 (IGFBP3) activity but increased IGFBP2 levels. Serum GH concentrations were significantly elevated compared with control pigs. GHR-KO pigs had a normal birth weight. Growth retardation became significant at the age of five weeks. At the age of six months, the body weight of GHR-KO pigs was reduced by 60% compared with controls. Most organ weights of GHR-KO pigs were reduced proportionally to body weight. However, the weights of liver, kidneys, and heart were disproportionately reduced, while the relative brain weight was almost doubled. GHR-KO pigs had a markedly increased percentage of total body fat relative to body weight and displayed transient juvenile hypoglycemia along with decreased serum triglyceride and cholesterol levels. Analysis of insulin receptor related signaling in the liver of adult fasted pigs revealed increased phosphorylation of IRS1 and PI3K. In agreement with the loss of GHR, phosphorylation of STAT5 was significantly reduced. In contrast, phosphorylation of JAK2 was significantly increased, possibly due to the increased serum leptin levels and increased hepatic leptin receptor expression and activation in GHR-KO pigs. In addition, increased mTOR phosphorylation was observed in GHR-KO liver samples, and phosphorylation studies of downstream substrates suggested the activation of mainly mTOR complex 2.
Conclusion: GHR-KO pigs resemble the pathophysiology of LS and are an interesting model for mechanistic studies and treatment trials.
Keywords: Dwarfism; Growth hormone receptor; Hypoglycemia; Insulin-like growth factor 1; Laron syndrome; Pig model; Signaling.
Copyright © 2018 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures
References
-
- Laron Z., Pertzelan A., Mannheimer S. Genetic pituitary dwarfism with high serum concentation of growth hormone–a new inborn error of metabolism? Israel Journal of Medical Sciences. 1966;2:152–155. - PubMed
-
- Laron Z. Clinical evidence of growth hormone resistance in patients with Laron syndrome. In: Laron Z., Kopchick J.J., editors. Laron Syndrome - from Man to Mouse. Springer; 2011. pp. 21–25.
-
- Laron Z. Laron syndrome (primary growth hormone resistance or insensitivity): the personal experience 1958–2003. The Journal of Clinical Endocrinology and Metabolism. 2004;89:1031–1044. - PubMed
-
- Laron Z. Epilogue: the future of Laron syndrome — the need for changes. Growth Hormone & IGF Research. 2016;28:79–80. - PubMed
-
- Guevara-Aguirre J., Rosenbloom A.L., Fielder P.J., Diamond F.B., Jr., Rosenfeld R.G. Growth hormone receptor deficiency in Ecuador: clinical and biochemical phenotype in two populations. The Journal of Clinical Endocrinology and Metabolism. 1993;76:417–423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

