Rapid Disinhibition by Adjustment of PV Intrinsic Excitability during Whisker Map Plasticity in Mouse S1
- PMID: 29678876
- PMCID: PMC5956988
- DOI: 10.1523/JNEUROSCI.3628-17.2018
Rapid Disinhibition by Adjustment of PV Intrinsic Excitability during Whisker Map Plasticity in Mouse S1
Abstract
Rapid plasticity of layer (L) 2/3 inhibitory circuits is an early step in sensory cortical map plasticity, but its cellular basis is unclear. We show that, in mice of either sex, 1 d whisker deprivation drives the rapid loss of L4-evoked feedforward inhibition and more modest loss of feedforward excitation in L2/3 pyramidal (PYR) cells, increasing the excitation-inhibition conductance ratio. Rapid disinhibition was due to reduced L4-evoked spiking by L2/3 parvalbumin (PV) interneurons, caused by reduced PV intrinsic excitability. This included elevated PV spike threshold, which is associated with an increase in low-threshold, voltage-activated delayed rectifier (presumed Kv1) and A-type potassium currents. Excitatory synaptic input and unitary inhibitory output of PV cells were unaffected. Functionally, the loss of feedforward inhibition and excitation was precisely coordinated in L2/3 PYR cells, so that peak feedforward synaptic depolarization remained stable. Thus, the rapid plasticity of PV intrinsic excitability offsets early weakening of excitatory circuits to homeostatically stabilize synaptic potentials in PYR cells of sensory cortex.SIGNIFICANCE STATEMENT Inhibitory circuits in cerebral cortex are highly plastic, but the cellular mechanisms and functional importance of this plasticity are incompletely understood. We show that brief (1 d) sensory deprivation rapidly weakens parvalbumin (PV) inhibitory circuits by reducing the intrinsic excitability of PV neurons. This involved a rapid increase in voltage-gated potassium conductances that control near-threshold spiking excitability. Functionally, the loss of PV-mediated feedforward inhibition in L2/3 pyramidal cells was precisely balanced with the separate loss of feedforward excitation, resulting in a net homeostatic stabilization of synaptic potentials. Thus, rapid plasticity of PV intrinsic excitability implements network-level homeostasis to stabilize synaptic potentials in sensory cortex.
Keywords: PV neuron; feedforward inhibition; homeostasis; intrinsic excitability; plasticity; sensory cortex.
Copyright © 2018 the authors 0270-6474/18/384749-13$15.00/0.
Figures


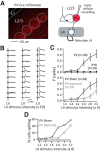
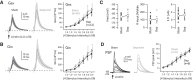
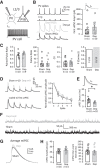
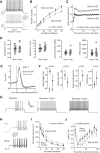


Similar articles
-
Cellular mechanisms for response heterogeneity among L2/3 pyramidal cells in whisker somatosensory cortex.J Neurophysiol. 2014 Jul 15;112(2):233-48. doi: 10.1152/jn.00848.2013. Epub 2014 Apr 16. J Neurophysiol. 2014. PMID: 24740854 Free PMC article.
-
Parallel regulation of feedforward inhibition and excitation during whisker map plasticity.Neuron. 2011 Dec 8;72(5):819-31. doi: 10.1016/j.neuron.2011.09.008. Neuron. 2011. PMID: 22153377 Free PMC article.
-
Rapid homeostasis by disinhibition during whisker map plasticity.Proc Natl Acad Sci U S A. 2014 Jan 28;111(4):1616-21. doi: 10.1073/pnas.1312455111. Epub 2014 Jan 13. Proc Natl Acad Sci U S A. 2014. PMID: 24474788 Free PMC article.
-
Multiple shared mechanisms for homeostatic plasticity in rodent somatosensory and visual cortex.Philos Trans R Soc Lond B Biol Sci. 2017 Mar 5;372(1715):20160157. doi: 10.1098/rstb.2016.0157. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28093551 Free PMC article. Review.
-
Parvalbumin-Positive Interneurons Regulate Cortical Sensory Plasticity in Adulthood and Development Through Shared Mechanisms.Front Neural Circuits. 2022 May 6;16:886629. doi: 10.3389/fncir.2022.886629. eCollection 2022. Front Neural Circuits. 2022. PMID: 35601529 Free PMC article. Review.
Cited by
-
Activation of M1 cholinergic receptors in mouse somatosensory cortex enhances information processing and detection behaviour.Commun Biol. 2024 Jan 2;7(1):3. doi: 10.1038/s42003-023-05699-w. Commun Biol. 2024. PMID: 38168628 Free PMC article.
-
A circuit mechanism for independent modulation of excitatory and inhibitory firing rates after sensory deprivation.Proc Natl Acad Sci U S A. 2022 Aug 9;119(32):e2116895119. doi: 10.1073/pnas.2116895119. Epub 2022 Aug 4. Proc Natl Acad Sci U S A. 2022. PMID: 35925891 Free PMC article.
-
Unreliable homeostatic action potential broadening in cultured dissociated neurons.bioRxiv [Preprint]. 2025 May 15:2025.05.09.653135. doi: 10.1101/2025.05.09.653135. bioRxiv. 2025. PMID: 40463064 Free PMC article. Preprint.
-
Behavioral and Neural Bases of Tactile Shape Discrimination Learning in Head-Fixed Mice.Neuron. 2020 Dec 9;108(5):953-967.e8. doi: 10.1016/j.neuron.2020.09.012. Epub 2020 Sep 30. Neuron. 2020. PMID: 33002411 Free PMC article.
-
Molecular states underlying neuronal cell type development and plasticity in the whisker cortex.bioRxiv [Preprint]. 2024 Oct 7:2024.10.07.617106. doi: 10.1101/2024.10.07.617106. bioRxiv. 2024. Update in: PLoS Biol. 2025 May 14;23(5):e3003176. doi: 10.1371/journal.pbio.3003176. PMID: 39416021 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
