Potassium is a trigger for conformational change in the fusion spike of an enveloped RNA virus
- PMID: 29678879
- PMCID: PMC6028977
- DOI: 10.1074/jbc.RA118.002494
Potassium is a trigger for conformational change in the fusion spike of an enveloped RNA virus
Erratum in
-
Correction: Potassium is a trigger for conformational change in the fusion spike of an enveloped RNA virus.J Biol Chem. 2019 Feb 15;294(7):2579. doi: 10.1074/jbc.AAC119.007718. J Biol Chem. 2019. PMID: 30765512 Free PMC article. No abstract available.
Abstract
Many enveloped viruses enter cells through the endocytic network, from which they must subsequently escape through fusion of viral and endosomal membranes. This membrane fusion is mediated by virus-encoded spikes that respond to the dynamic endosomal environment, which triggers conformational changes in the spikes that initiate the fusion process. Several fusion triggers have been identified and include pH, membrane composition, and endosome-resident proteins, and these cues dictate when and where viral fusion occurs. We recently reported that infection with an enveloped bunyavirus requires elevated potassium ion concentrations [K+], controlled by cellular K+ channels, that are encountered during viral transit through maturing endosomes. Here we reveal the molecular basis for the K+ requirement of bunyaviruses through the first direct visualization of a member of the Nairoviridae family, namely Hazara virus (HAZV), using cryo-EM. Using cryo-electron tomography, we observed HAZV spike glycoproteins within infectious HAZV particles exposed to both high and low [K+], which showed that exposure to K+ alone results in dramatic changes to the ultrastructural architecture of the virion surface. In low [K+], the spikes adopted a compact conformation arranged in locally ordered arrays, whereas, following exposure to high [K+], the spikes became extended, and spike-membrane interactions were observed. Viruses exposed to high [K+] also displayed enhanced infectivity, thus identifying K+ as a newly defined trigger that helps promote viral infection. Finally, we confirmed that K+ channel blockers are inhibitory to HAZV infection, highlighting the potential of K+ channels as anti-bunyavirus targets.
Keywords: conformational change; electron tomography; fusion protein; ion channel; potassium transport; virus entry; virus structure.
© 2018 Punch et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
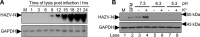



References
-
- Adams M. J., Lefkowitz E. J., King A. M. Q., Harrach B., Harrison R. L., Knowles N. J., Kropinski A. M., Krupovic M., Kuhn J. H., Mushegian A. R., Nibert M., Sabanadzovic S., Sanfaçon H., Siddell S. G., Simmonds P., et al. (2017) Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2017). Arch. Virol. 162, 2505–2538 10.1007/s00705-017-3358-5 - DOI - PubMed
-
- Suleiman M. N., Muscat-Baron J. M., Harries J. R., Satti A. G., Platt G. S., Bowen E. T., and Simpson D. I. (1980) Congo/Crimean haemorrhagic fever in Dubai: an outbreak at the Rashid Hospital. Lancet 2, 939–941 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

