Protocol for a phase III, non-inferiority, randomised comparison of a new fibrinogen concentrate versus cryoprecipitate for treating acquired hypofibrinogenaemia in bleeding cardiac surgical patients: the FIBRES trial
- PMID: 29678987
- PMCID: PMC5914770
- DOI: 10.1136/bmjopen-2017-020741
Protocol for a phase III, non-inferiority, randomised comparison of a new fibrinogen concentrate versus cryoprecipitate for treating acquired hypofibrinogenaemia in bleeding cardiac surgical patients: the FIBRES trial
Abstract
Introduction: Coagulopathic bleeding is a serious complication of cardiac surgery to which an important contributor is acquired hypofibrinogenaemia (plasma fibrinogen <1.5-2.0 g/L). The standard intervention for acquired hypofibrinogenaemia is cryoprecipitate, but purified fibrinogen concentrates are also available. There is little comparative data between the two therapies and randomised trials are needed.
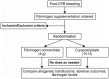
Methods and analysis: FIBrinogen REplenishment in Surgery (FIBRES) is a multicentre, randomised (1:1), active-control, single-blinded, phase III trial in adult cardiac surgical patients experiencing clinically significant bleeding related to acquired hypofibrinogenaemia. The primary objective is to demonstrate that fibrinogen concentrate (Octafibrin/Fibryga; Octapharma) is non-inferior to cryoprecipitate. All patients for whom fibrinogen supplementation is ordered by the clinical team within 24 hours of cardiopulmonary bypass will receive 4 g of fibrinogen concentrate or 10 units of cryoprecipitate (dose-equivalent to 4 g), based on random allocation and deferred consent. The primary outcome is total red cell, platelet and plasma transfusions administered within 24 hours of bypass. Secondary outcomes include major bleeding, fibrinogen levels and adverse events within 28 days. Enrolment of 1200 patients will provide >90% power to demonstrate non-inferiority. One preplanned interim analysis will include 600 patients. The pragmatic design and treatment algorithm align with standard practice, aiding adherence and generalisability.
Ethics and dissemination: The study is approved by the local research ethics board and will be conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines and regulatory requirements. Patient consent prior to treatment is waived, as per criteria in the Tri-Council Policy Statement. Results will be published in the scientific/medical literature, and at international congresses. Non-inferiority of purified fibrinogen concentrate would support its use in acquired hypofibrinogenaemia. The results are likely to improve care for cardiac surgical patients experiencing significant bleeding, an understudied yet high-risk population.
Trial registration number: NCT03037424; Pre-results.
Keywords: adult surgery; bleeding disorders and coagulopathies; blood bank & transfusion medicine; cardiac surgery; clinical trials; haematology.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: KK has received support for research and/or honoraria from Octapharma. JC has received support for research through peer-reviewed grants from Canadian Blood Services. NH is the Research Director for the McMaster Centre for Transfusion Research, which receives funding support from Canadian Blood Services and Health Canada. The remaining authors have no competing interests or conflicts to declare.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical