Nucleus accumbens controls wakefulness by a subpopulation of neurons expressing dopamine D1 receptors
- PMID: 29679009
- PMCID: PMC5910424
- DOI: 10.1038/s41467-018-03889-3
Nucleus accumbens controls wakefulness by a subpopulation of neurons expressing dopamine D1 receptors
Abstract
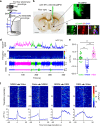
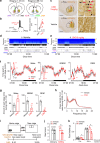
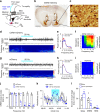
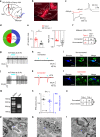
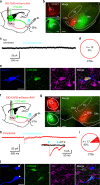
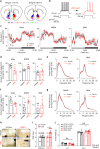
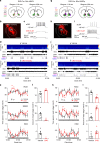
Nucleus accumbens (NAc) is involved in behaviors that depend on heightened wakefulness, but its impact on arousal remains unclear. Here, we demonstrate that NAc dopamine D1 receptor (D1R)-expressing neurons are essential for behavioral arousal. Using in vivo fiber photometry in mice, we find arousal-dependent increases in population activity of NAc D1R neurons. Optogenetic activation of NAc D1R neurons induces immediate transitions from non-rapid eye movement sleep to wakefulness, and chemogenetic stimulation prolongs arousal, with decreased food intake. Patch-clamp, tracing, immunohistochemistry, and electron microscopy reveal that NAc D1R neurons project to the midbrain and lateral hypothalamus, and might disinhibit midbrain dopamine neurons and lateral hypothalamus orexin neurons. Photoactivation of terminals in the midbrain and lateral hypothalamus is sufficient to induce wakefulness. Silencing of NAc D1R neurons suppresses arousal, with increased nest-building behaviors. Collectively, our data indicate that NAc D1R neuron circuits are essential for the induction and maintenance of wakefulness.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Dopamine D1-receptor-expressing pathway from the nucleus accumbens to ventral pallidum-mediated sevoflurane anesthesia in mice.CNS Neurosci Ther. 2023 Nov;29(11):3364-3377. doi: 10.1111/cns.14267. Epub 2023 May 19. CNS Neurosci Ther. 2023. PMID: 37208941 Free PMC article.
-
Dopamine D1 Receptor in the Nucleus Accumbens Modulates the Emergence from Propofol Anesthesia in Rat.Neurochem Res. 2021 Jun;46(6):1435-1446. doi: 10.1007/s11064-021-03284-3. Epub 2021 Mar 8. Neurochem Res. 2021. PMID: 33683630
-
Striatal neurons expressing dopamine D1 receptor promote wakefulness in mice.Curr Biol. 2022 Feb 7;32(3):600-613.e4. doi: 10.1016/j.cub.2021.12.026. Epub 2022 Jan 11. Curr Biol. 2022. PMID: 35021048
-
The First Alcohol Drink Triggers mTORC1-Dependent Synaptic Plasticity in Nucleus Accumbens Dopamine D1 Receptor Neurons.J Neurosci. 2016 Jan 20;36(3):701-13. doi: 10.1523/JNEUROSCI.2254-15.2016. J Neurosci. 2016. PMID: 26791202 Free PMC article.
-
The control of sleep and wakefulness by mesolimbic dopamine systems.Neurosci Res. 2017 May;118:66-73. doi: 10.1016/j.neures.2017.04.008. Epub 2017 Apr 20. Neurosci Res. 2017. PMID: 28434991 Review.
Cited by
-
Reactivating a positive feedback loop VTA-BLA-NAc circuit associated with positive experience ameliorates the attenuated reward sensitivity induced by chronic stress.Neurobiol Stress. 2021 Jul 24;15:100370. doi: 10.1016/j.ynstr.2021.100370. eCollection 2021 Nov. Neurobiol Stress. 2021. PMID: 34381852 Free PMC article.
-
Insomnia-related rodent models in drug discovery.Acta Pharmacol Sin. 2024 Sep;45(9):1777-1792. doi: 10.1038/s41401-024-01269-w. Epub 2024 Apr 26. Acta Pharmacol Sin. 2024. PMID: 38671193 Review.
-
Nucleus accumbens dynamics in food reward seeking and regulation by sleep.Transl Psychiatry. 2025 Jul 1;15(1):219. doi: 10.1038/s41398-025-03442-z. Transl Psychiatry. 2025. PMID: 40593485 Free PMC article.
-
Ventral subiculum promotes wakefulness through several pathways in male mice.Neuropsychopharmacology. 2024 Aug;49(9):1468-1480. doi: 10.1038/s41386-024-01875-6. Epub 2024 May 11. Neuropsychopharmacology. 2024. PMID: 38734818 Free PMC article.
-
Whole-Brain Monosynaptic Afferent Projections to the Cholecystokinin Neurons of the Suprachiasmatic Nucleus.Front Neurosci. 2018 Nov 5;12:807. doi: 10.3389/fnins.2018.00807. eCollection 2018. Front Neurosci. 2018. PMID: 30455627 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

