Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model
- PMID: 29679025
- PMCID: PMC5910383
- DOI: 10.1038/s41598-018-24892-0
Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model
Abstract
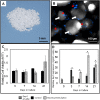
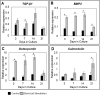
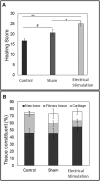
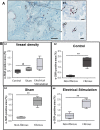
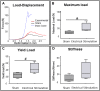
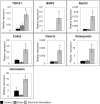
Bone Tissue engineering (BTE) has recently been introduced as an alternative to conventional treatments for large non-healing bone defects. BTE approaches mimic autologous bone grafts, by combining cells, scaffold, and growth factors, and have the added benefit of being able to manipulate these constituents to optimize healing. Electrical stimulation (ES) has long been used to successfully treat non-healing fractures and has recently been shown to stimulate bone cells to migrate, proliferate, align, differentiate, and adhere to bio compatible scaffolds, all cell behaviors that could improve BTE treatment outcomes. With the above in mind we performed in vitro experiments and demonstrated that exposing Mesenchymal Stem Cells (MSC) + scaffold to ES for 3 weeks resulted in significant increases in osteogenic differentiation. Then in in vivo experiments, for the first time, we demonstrated that exposing BTE treated rat femur large defects to ES for 8 weeks, caused improved healing, as indicated by increased bone formation, strength, vessel density, and osteogenic gene expression. Our results demonstrate that ES significantly increases osteogenic differentiation in vitro and that this effect is translated into improved healing in vivo. These findings support the use of ES to help BTE treatments achieve their full therapeutic potential.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
The effect of autologous bone marrow stromal cells differentiated on scaffolds for canine tibial bone reconstruction.Biotech Histochem. 2015;90(7):516-28. doi: 10.3109/10520295.2014.983547. Epub 2015 May 21. Biotech Histochem. 2015. PMID: 25994048
-
Guided bone regeneration in pig calvarial bone defects using autologous mesenchymal stem/progenitor cells - a comparison of different tissue sources.J Craniomaxillofac Surg. 2012 Jun;40(4):310-20. doi: 10.1016/j.jcms.2011.05.004. Epub 2011 Jun 30. J Craniomaxillofac Surg. 2012. PMID: 21723141
-
Electrical stimulation in bone tissue engineering treatments.Eur J Trauma Emerg Surg. 2020 Apr;46(2):231-244. doi: 10.1007/s00068-020-01324-1. Epub 2020 Feb 20. Eur J Trauma Emerg Surg. 2020. PMID: 32078704 Free PMC article. Review.
-
Pretreatment of Mesenchymal Stem Cells with Electrical Stimulation as a Strategy to Improve Bone Tissue Engineering Outcomes.Cells. 2023 Aug 26;12(17):2151. doi: 10.3390/cells12172151. Cells. 2023. PMID: 37681884 Free PMC article.
-
Clinical Applications of Cell-Scaffold Constructs for Bone Regeneration Therapy.Cells. 2021 Oct 8;10(10):2687. doi: 10.3390/cells10102687. Cells. 2021. PMID: 34685667 Free PMC article. Review.
Cited by
-
Effects of electrical stimulation with alternating fields on the osseointegration of titanium implants in the rabbit tibia - a pilot study.Front Bioeng Biotechnol. 2024 Jul 24;12:1395715. doi: 10.3389/fbioe.2024.1395715. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39113790 Free PMC article.
-
Multifunctional Scaffolds and Synergistic Strategies in Tissue Engineering and Regenerative Medicine.Pharmaceutics. 2021 May 26;13(6):792. doi: 10.3390/pharmaceutics13060792. Pharmaceutics. 2021. PMID: 34073311 Free PMC article. Review.
-
Recent Advances on Stimuli-Responsive Hydrogels Based on Tissue-Derived ECMs and Their Components: Towards Improving Functionality for Tissue Engineering and Controlled Drug Delivery.Polymers (Basel). 2021 Sep 25;13(19):3263. doi: 10.3390/polym13193263. Polymers (Basel). 2021. PMID: 34641079 Free PMC article. Review.
-
Effects of Varied Stimulation Parameters on Adipose-Derived Stem Cell Response to Low-Level Electrical Fields.Ann Biomed Eng. 2021 Dec;49(12):3401-3411. doi: 10.1007/s10439-021-02875-z. Epub 2021 Oct 26. Ann Biomed Eng. 2021. PMID: 34704163 Free PMC article.
-
Electrical Stimulation Activates Fibroblasts through the Elevation of Intracellular Free Ca2+: Potential Mechanism of Pelvic Electrical Stimulation Therapy.Biomed Res Int. 2019 Apr 21;2019:7387803. doi: 10.1155/2019/7387803. eCollection 2019. Biomed Res Int. 2019. PMID: 31139648 Free PMC article.
References
-
- Giannoudis PV, Atkins R. Management of long-bone non-unions. Injury. 2007;38(Suppl 2):S1–2. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

