A probiotic modulates the microbiome and immunity in multiple sclerosis
- PMID: 29679417
- PMCID: PMC6181139
- DOI: 10.1002/ana.25244
A probiotic modulates the microbiome and immunity in multiple sclerosis
Abstract
Objective: Effect of a probiotic on the gut microbiome and peripheral immune function in healthy controls and relapsing-remitting multiple sclerosis (MS) patients.
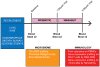
Methods: MS patients (N = 9) and controls (N = 13) were orally administered a probiotic containing Lactobacillus, Bifidobacterium, and Streptococcus twice-daily for two months. Blood and stool specimens were collected at baseline, after completion of the 2-month treatment, and 3 months after discontinuation of therapy. Frozen peripheral blood mononuclear cells (PBMCs) were used for immune cell profiling. Stool samples were used for 16S rRNA profiling and metabolomics.
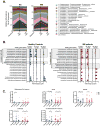
Results: Probiotic administration increased the abundance of several taxa known to be depleted in MS such as Lactobacillus. We found that probiotic use decreased the abundance of taxa previously associated with dysbiosis in MS, including Akkermansia and Blautia. Predictive metagenomic analysis revealed a decrease in the abundance of several KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways associated with altered gut microbiota function in MS patients, such as methane metabolism, following probiotic supplementation. At the immune level, probiotic administration induced an anti-inflammatory peripheral immune response characterized by decreased frequency of inflammatory monocytes, decreased mean fluorescence intensity (MFI) of CD80 on classical monocytes, as well as decreased human leukocyte antigen (HLA) D related MFI on dendritic cells. Probiotic administration was also associated with decreased expression of MS risk allele HLA-DQA1 in controls. Probiotic-induced increase in abundance of Lactobacillus and Bifidobacterium was associated with decreased expression of MS risk allele HLA.DPB1 in controls.
Interpretation: Our results suggest that probiotics could have a synergistic effect with current MS therapies. Ann Neurol 2018.
© 2018 American Neurological Association.
Conflict of interest statement
This study was supported in part by Teva Neuroscience (Teva manufactures the drug glatiramer acetate used in this study).
Figures




References
-
- Zhang D, Jia H, Feng Q, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015:1–13. - PubMed
-
- Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, et al. Role of Gut Commensal Microflora in the Development of Experimental Autoimmune Encephalomyelitis. The Journal of Immunology. 2009;183(10):6041–6050. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

