Influence of Human Milk and Parenteral Lipid Emulsions on Serum Fatty Acid Profiles in Extremely Preterm Infants
- PMID: 29679529
- PMCID: PMC6437763
- DOI: 10.1002/jpen.1172
Influence of Human Milk and Parenteral Lipid Emulsions on Serum Fatty Acid Profiles in Extremely Preterm Infants
Abstract
Background: Infants born prematurely are at risk of a deficiency in ω-6 and ω-3 long-chain polyunsaturated fatty acids (LC-PUFAs) arachidonic acid (AA) and docosahexaenoic acid (DHA). We investigated how fatty acids from breast milk and parenteral lipid emulsions shape serum LC-PUFA profiles in extremely preterm infants during early perinatal life.
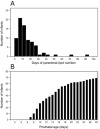
Methods: Ninety infants born < 28 weeks gestational age were randomized to receive parenteral lipids with or without the ω-3 LC-PUFAs eicosapentaenoic acid (EPA) and DHA (SMOFlipid: Fresenius Kabi, Uppsala, Sweden, or Clinoleic: Baxter Medical AB, Kista, Sweden, respectively). The fatty acid composition of infant serum phospholipids was determined from birth to postmenstrual age 40 weeks, and in mother's milk total lipids on postnatal day 7. Enteral and parenteral intake of LC-PUFAs was correlated with levels in infant serum.
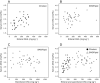
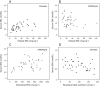
Results: Infants administered parenteral ω-3 LC-PUFAs received 4.4 and 19.3 times more DHA and EPA, respectively, over the first 2 weeks of life. Parenteral EPA but not DHA correlated with levels in infant serum. We found linear relationships between dietary EPA and DHA and infant serum levels in the Clinoleic (Baxter Medical AB) group. The volume of administered SMOFlipid (Fresenius Kabi) was inversely correlated with serum AA, whereas Clinoleic (Baxter Medical AB) inversely correlated with serum EPA and DHA.
Conclusions: There appears to be no or low correlation between the amount of DHA administered parenterally and levels measured in serum. Whether this observation reflects serum phospholipid fraction only or truly represents the amount of accreted DHA needs to be investigated. None of the parenteral lipid emulsions satisfactorily maintained high levels of both ω-6 and ω-3 LC-PUFAs in infant serum.
Keywords: arachidonic acid; docosahexaenoic acid (DHA); extremely preterm; human milk; long-chain polyunsaturated fatty acids (LC-PUFA); parenteral nutrition.
© 2018 The Authors. Journal of Parenteral and Enteral Nutrition published by American Society for Parenteral and Enteral Nutrition.
Figures
References
-
- Driscoll DF, Bistrian BR, Demmelmair H, Koletzko B. Pharmaceutical and clinical aspects of parenteral lipid emulsions in neonatology. Clin Nutr 2008;27:497‐503. - PubMed
-
- Fang PC, Kuo HK, Huang CB, Ko TY, Chen CC, Chung MY. The effect of supplementation of docosahexaenoic acid and arachidonic acid on visual acuity and neurodevelopment in larger preterm infants. Chang Gung Med J 2005;28:708‐715. - PubMed
-
- Wang Q, Cui Q, Yan C. The effect of supplementation of long‐chain polyunsaturated fatty acids during lactation on neurodevelopmental outcomes of preterm infant from infancy to school age: a systematic review and meta‐analysis. Pediatr Neurol 2016;59:54‐61.e1. - PubMed
-
- Lapillonne A. Enteral and parenteral lipid requirements of preterm infants. World Rev Nutr Diet 2014;110:82‐98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

