MYH9: Structure, functions and role of non-muscle myosin IIA in human disease
- PMID: 29679756
- PMCID: PMC5970098
- DOI: 10.1016/j.gene.2018.04.048
MYH9: Structure, functions and role of non-muscle myosin IIA in human disease
Abstract
The MYH9 gene encodes the heavy chain of non-muscle myosin IIA, a widely expressed cytoplasmic myosin that participates in a variety of processes requiring the generation of intracellular chemomechanical force and translocation of the actin cytoskeleton. Non-muscle myosin IIA functions are regulated by phosphorylation of its 20 kDa light chain, of the heavy chain, and by interactions with other proteins. Variants of MYH9 cause an autosomal-dominant disorder, termed MYH9-related disease, and may be involved in other conditions, such as chronic kidney disease, non-syndromic deafness, and cancer. This review discusses the structure of the MYH9 gene and its protein, as well as the regulation and physiologic functions of non-muscle myosin IIA with particular reference to embryonic development. Moreover, the review focuses on current knowledge about the role of MYH9 variants in human disease.
Keywords: Actin-myosin cytoskeleton; Cell-cell adhesion; Class II myosin; Deafness; Inherited thrombocytopenia; Kidney disease; MYH9 gene; MYH9-related disease; Mouse models; Non-muscle myosin; Tumor suppressor.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

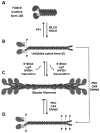
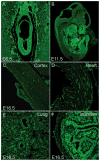
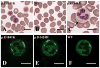
References
-
- Adelstein RS, Conti MA. Phosphorylation of platelet myosin increases actin-activated myosin ATPase activity. Nature. 1975;256:597–598. - PubMed
-
- Badirou I, Pan J, Souquere S, Legrand C, Pierron G, Wang A, Eckly A, Roy A, Gachet C, Vainchenker W, Chang Y, Léon C. Distinct localizations and roles of non-muscle myosin II during proplatelet formation and platelet release. J Thromb Haemost. 2015;13:851–859. - PubMed
-
- Balduini A, Pallotta I, Malara A, Lova P, Pecci A, Viarengo G, Balduini CL, Torti M. Adhesive receptors, extracellular proteins and myosin IIA orchestrate proplatelet formation by human megakaryocytes. J Thromb Haemost. 2008;6:1900–1907. - PubMed
-
- Balduini CL, Pecci A, Noris P. Diagnosis and management of inherited thrombocytopenias. Semin Thromb Hemost. 2013;39:161–171. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

