Estrogen receptor beta signaling inhibits PDGF induced human airway smooth muscle proliferation
- PMID: 29680290
- PMCID: PMC6120801
- DOI: 10.1016/j.mce.2018.04.007
Estrogen receptor beta signaling inhibits PDGF induced human airway smooth muscle proliferation
Abstract
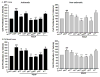
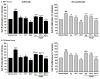
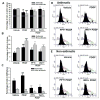
Airway smooth muscle (ASM) cell hyperplasia driven by persistent inflammation is a hallmark feature of remodeling in asthma. Sex steroid signaling in the lungs is of considerable interest, given epidemiological data showing more asthma in pre-menopausal women and aging men. Our previous studies demonstrated that estrogen receptor (ER) expression increases in asthmatic human ASM; however, very limited data are available regarding differential roles of ERα vs. ERβ isoforms in human ASM cell proliferation. In this study, we evaluated the effect of selective ERα and ERβ modulators on platelet-derived growth factor (PDGF)-stimulated ASM proliferation and the mechanisms involved. Asthmatic and non-asthmatic primary human ASM cells were treated with PDGF, 17β-estradiol, ERα-agonist and/or ERβ-agonist and/or G-protein-coupled estrogen receptor 30 (GPR30/GPER) agonist and proliferation was measured using MTT and CyQuant assays followed by cell cycle analysis. Transfection of small interfering RNA (siRNA) ERα and ERβ significantly altered the human ASM proliferation. The specificity of siRNA transfection was confirmed by Western blot analysis. Gene and protein expression of cell cycle-related antigens (PCNA and Ki67) and C/EBP were measured by RT-PCR and Western analysis, along with cell signaling proteins. PDGF significantly increased ASM proliferation in non-asthmatic and asthmatic cells. Treatment with PPT showed no significant effect on PDGF-induced proliferation, whereas WAY interestingly suppressed proliferation via inhibition of ERK1/2, Akt, and p38 signaling. PDGF-induced gene expression of PCNA, Ki67 and C/EBP in human ASM was significantly lower in cells pre-treated with WAY. Furthermore, WAY also inhibited PDGF-activated PCNA, C/EBP, cyclin-D1, and cyclin-E. Overall, we demonstrate ER isoform-specific signaling in the context of ASM proliferation. Activation of ERβ can diminish remodeling in human ASM by inhibiting pro-proliferative signaling pathways, and may point to a novel perception for blunting airway remodeling.
Keywords: Asthma; ERα receptor; Estrogen; Lung; PCNA; Sex steroids.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures

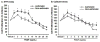




References
-
- Lazaar AL, Panettieri RA., Jr Airway smooth muscle: a modulator of airway remodeling in asthma. J Allergy Clin Immunol. 2005;116:488–95. quiz 496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

