Novel PET and Near Infrared Imaging Probes for the Specific Detection of Bacterial Infections Associated With Cardiac Devices
- PMID: 29680350
- PMCID: PMC6186207
- DOI: 10.1016/j.jcmg.2018.02.011
Novel PET and Near Infrared Imaging Probes for the Specific Detection of Bacterial Infections Associated With Cardiac Devices
Abstract
Objectives: The aim of this study was to develop imaging agents to detect early stage infections in implantable cardiac devices.
Background: Bacteria ingest maltodextrins through the specific maltodextrin transporter. We developed probes conjugated with either a fluorescent dye (maltohexaose fluorescent dye probe [MDP]) or a F-18 (F18 fluoromaltohexaose) and determined their usefulness in a model of infections associated with implanted cardiac devices.
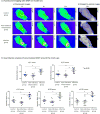
Methods: Stainless steel mock-ups of medical devices were implanted subcutaneously in rats. On post-operative day 4, animals were injected with either Staphylococcus aureus around the mock-ups to induce a relatively mild infection or oil of turpentine to induce noninfectious inflammation. Animals with a sterile implant were used as control subjects. On post-operative day 6, either the MDP or F18 fluoromaltohexaose was injected intravenously, and the animals were scanned with the appropriate imaging device. Additional positron emission tomography imaging studies were performed with F18-fluorodeoxyglucose as a comparison of the specificity of our probes (n = 5 to 9 per group).
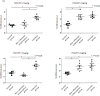
Results: The accumulation of the MDP in the infected rats was significantly increased at 1 h after injection when compared with the control and noninfectious inflammation groups (intensity ratio 1.54 ± 0.07 vs. 1.26 ± 0.04 and 1.20 ± 0.05, respectively; p < 0.05) and persisted for more than 24 h. In positron emission tomography imaging, both F18 fluoromaltohexaose and F18 fluorodeoxyglucose significantly accumulated in the infected area 30 min after the injection (maximum standard uptake value ratio 4.43 ± 0.30 and 4.87 ± 0.28, respectively). In control rats, there was no accumulation of imaging probes near the device. In the noninfectious inflammation rats, no significant accumulation was observed with F18 fluoromaltohexaose, but F18 fluorodeoxyglucose accumulated in the mock-up area (maximum standard uptake value 2.53 ± 0.39 vs. 4.74 ± 0.46, respectively; p < 0.05).
Conclusions: Our results indicate that maltohexaose-based imaging probes are potentially useful for the specific and sensitive diagnosis of infections associated with implantable cardiac devices.
Keywords: PET; bacterial imaging; medical device infections; optical imaging.
Copyright © 2019 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures
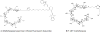



Comment in
-
Targeted Bacteria-Specific 18F-Fluoro-Maltohexaose But Not FDG PET Distinguishes Infection From Inflammation.JACC Cardiovasc Imaging. 2019 May;12(5):887-889. doi: 10.1016/j.jcmg.2018.03.008. Epub 2018 Apr 18. JACC Cardiovasc Imaging. 2019. PMID: 29680348 No abstract available.
References
-
- Olsen NT, De Backer O, Thyregod HG, et al. Prosthetic valve endocarditis after transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2015;8 - PubMed
-
- Greenspon AJ, Patel JD, Lau E, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. Journal of the American College of Cardiology. 2011;58:1001–6. - PubMed
-
- Tarakji KG, Wilkoff BL. Management of cardiac implantable electronic device infections: the challenges of understanding the scope of the problem and its associated mortality. Expert review of cardiovascular therapy. 2013;11:607–16. - PubMed
-
- Sarrazin JF, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. Journal of the American College of Cardiology. 2012;59:1616–25. - PubMed
-
- Nataloni M, Pergolini M, Rescigno G, Mocchegiani R. Prosthetic valve endocarditis. Journal of cardiovascular medicine. 2010;11:869–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

