Full and partial agonists evoke distinct structural changes in opening the muscle acetylcholine receptor channel
- PMID: 29680816
- PMCID: PMC5940249
- DOI: 10.1085/jgp.201711881
Full and partial agonists evoke distinct structural changes in opening the muscle acetylcholine receptor channel
Abstract
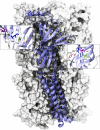
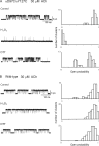
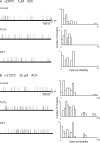
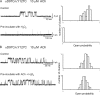
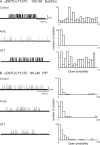
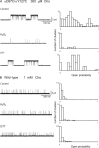
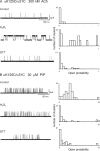
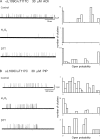
The muscle acetylcholine (ACh) receptor transduces a chemical into an electrical signal, but the efficiency of transduction, or efficacy, depends on the particular agonist. It is often presumed that full and partial agonists elicit the same structural changes after occupancy of their binding sites but with differing speed and efficiency. In this study, we tested the alternative hypothesis that full and partial agonists elicit distinct structural changes. To probe structural changes, we substituted cysteines for pairs of residues that are juxtaposed in the three-dimensional structure and recorded agonist-elicited single-channel currents before and after the addition of an oxidizing reagent. The results revealed multiple cysteine pairs for which agonist-elicited channel opening changes after oxidative cross-linking. Moreover, we found that the identity of the agonist determined whether cross-linking affects channel opening. For the αD97C/αY127C pair at the principal face of the subunit, cross-linking markedly suppressed channel opening by full but not partial agonists. Conversely, for the αD97C/αK125C pair, cross-linking impaired channel opening by the weak agonist choline but not other full or partial agonists. For the αT51C/αK125C pair, cross-linking enhanced channel opening by the full agonist ACh but not other full or partial agonists. At the complementary face of the subunit, cross-linking between pairs within the same β hairpin suppressed channel opening by ACh, whereas cross-linking between pairs from adjacent β hairpins was without effect for all agonists. In each case, the effects of cross-linking were reversed after addition of a reducing reagent, and receptors with single cysteine substitutions remained unaltered after addition of either oxidizing or reducing reagents. These findings show that, in the course of opening the receptor channel, different agonists elicit distinct structural changes.
© 2018 Sine and Mukhtasimova.
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

