Influenza Hemagglutinin Protein Stability, Activation, and Pandemic Risk
- PMID: 29681430
- PMCID: PMC6150828
- DOI: 10.1016/j.tim.2018.03.005
Influenza Hemagglutinin Protein Stability, Activation, and Pandemic Risk
Abstract
For decades, hemagglutinin (HA) protein structure and its refolding mechanism have served as a paradigm for understanding protein-mediated membrane fusion. HA trimers are in a high-energy state and are functionally activated by low pH. Over the past decade, HA stability (or the pH at which irreversible conformational changes are triggered) has emerged as an important determinant in influenza virus host range, infectivity, transmissibility, and human pandemic potential. Here, we review HA protein structure, assays to measure its stability, measured HA stability values, residues and mutations that regulate its stability, the effect of HA stability on interspecies adaptation and transmissibility, and mechanistic insights into this process. Most importantly, HA stabilization appears to be necessary for adapting emerging influenza viruses to humans.
Keywords: fusion glycoprotein; influenza A virus; interspecies adaptation; pandemic; virus entry; virus transmission.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures


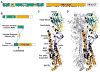



References
-
- Yoon SW, et al. Evolution and ecology of influenza A viruses. Curr Top Microbiol Immunol. 2014;385:359–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

