Differentiation of Human Pluripotent Stem Cells into Functional Endothelial Cells in Scalable Suspension Culture
- PMID: 29681541
- PMCID: PMC5995343
- DOI: 10.1016/j.stemcr.2018.03.017
Differentiation of Human Pluripotent Stem Cells into Functional Endothelial Cells in Scalable Suspension Culture
Abstract
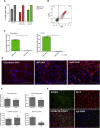
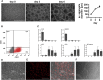
Endothelial cells (ECs) are involved in a variety of cellular responses. As multifunctional components of vascular structures, endothelial (progenitor) cells have been utilized in cellular therapies and are required as an important cellular component of engineered tissue constructs and in vitro disease models. Although primary ECs from different sources are readily isolated and expanded, cell quantity and quality in terms of functionality and karyotype stability is limited. ECs derived from human induced pluripotent stem cells (hiPSCs) represent an alternative and potentially superior cell source, but traditional culture approaches and 2D differentiation protocols hardly allow for production of large cell numbers. Aiming at the production of ECs, we have developed a robust approach for efficient endothelial differentiation of hiPSCs in scalable suspension culture. The established protocol results in relevant numbers of ECs for regenerative approaches and industrial applications that show in vitro proliferation capacity and a high degree of chromosomal stability.
Keywords: endothelial cells; hiPSC differentiation; scalable culture.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Albertson D.G., Collins C., McCormick F., Gray J.W. Chromosome aberrations in solid tumors. Nat. Genet. 2003;34:369–376. - PubMed
-
- Andrée B., Bela K., Horvath T., Lux M., Ramm R., Venturini L., Ciubotaru A., Zweigerdt R., Haverich A., Hilfiker A. Successful re-endothelialization of a perfusable biological vascularized matrix (BioVaM) for the generation of 3D artificial cardiac tissue. Basic Res. Cardiol. 2014;109:441. - PubMed
-
- Artandi S.E., Chang S., Lee S.L., Alson S., Gottlieb G.J., Chin L., DePinho R.A. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–645. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

