EDUCATIONAL SERIES IN CONGENITAL HEART DISEASE: Congenital left-sided heart obstruction
- PMID: 29681546
- PMCID: PMC5911774
- DOI: 10.1530/ERP-18-0016
EDUCATIONAL SERIES IN CONGENITAL HEART DISEASE: Congenital left-sided heart obstruction
Abstract
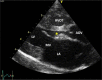
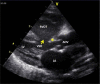
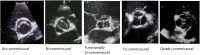
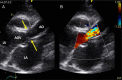
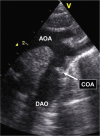
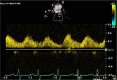
Congenital obstruction of the left ventricular outflow tract remains a significant problem and multilevel obstruction can often coexist. Obstruction can take several morphological forms and may involve the subvalvar, valvar or supravalvar portion of the aortic valve complex. Congenital valvar stenosis presenting in the neonatal period represents a spectrum of disorders ranging from the hypoplastic left heart syndrome to almost normal hearts. Treatment options vary dependent on the severity of the left ventricular outflow tract obstruction (LVOTO) and the variable degree of left ventricular hypoplasia as well as the associated lesions such as arch hypoplasia and coarctation.
Keywords: aortic stenosis; bicuspid aortic valve; coarctation of the aorta; congenital heart defects; left ventricular outflow tract obstruction.
© 2018 The authors.
Figures















References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

