Exploring hydroamination-cycloaddition-fragmentation sequences to access polycyclicguanidines and vinyl-2-aminoimidazoles
- PMID: 29681663
- PMCID: PMC5906054
- DOI: 10.1016/j.tet.2017.08.052
Exploring hydroamination-cycloaddition-fragmentation sequences to access polycyclicguanidines and vinyl-2-aminoimidazoles
Abstract
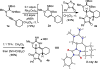
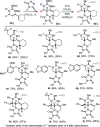
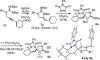
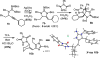
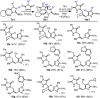
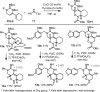
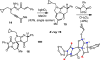
The intramolecular hydroamination of a guanidine on an eneyne unit affords a guanidine-substituted diene capable of reacting with dienophiles. These substrates undergo [4+2]-cycloaddition reactions to generate a series of complex cyclic- and spirocyclic-guanidines. Select substrates can further undergo a ring opening-elimination cascade that ultimately reveals a vinyl-2-aminoimidazole. As such this cascade reaction may find application in the synthesis of oroidin-type natural products and their analogues.
Keywords: 2-aminoimidazoles; Alkaloids; Cycloaddition; Heterocycle; Hydroamination.
Figures


References
-
- Harris TL, Worthington RJ, Melander C. Potent Small-Molecule Suppression of Oxacillin Resistance in Methicillin-Resistant Staphylococcus aureus. Angew Chem Int Ed. 2012;51(45):11254–11257. - PMC - PubMed
- Yeagley AA, Su Z, McCullough KD, Worthington RJ, Melander C. N-Substituted 2-aminoimidazole inhibitors of MRSA biofilm formation accessed through direct 1,3-bis(tert-butoxycarbonyl)guanidine cyclization. Org Biomol Chem. 2013;11(1):130–137. - PubMed
- Worthington RJ, Richards JJ, Melander C. Non-Microbicidal Control of Bacterial Biofilms with Small Molecules. Anti-Infect Agents. 2014;12(1):120–138.
-
- Al Mourabit A, Potier P. Sponge’s Molecular Diversity Through the Ambivalent Reactivity of 2-Aminoimidazole: A Universal Chemical Pathway to the Oroidin-Based Pyrrole-Imidazole Alkaloids and Their Palau’amine Congeners. European Journal of Organic Chemistry. 2001;2001(2):237–243.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
