DuCLOX-2/5 Inhibition Attenuates Inflammatory Response and Induces Mitochondrial Apoptosis for Mammary Gland Chemoprevention
- PMID: 29681851
- PMCID: PMC5897656
- DOI: 10.3389/fphar.2018.00314
DuCLOX-2/5 Inhibition Attenuates Inflammatory Response and Induces Mitochondrial Apoptosis for Mammary Gland Chemoprevention
Abstract
The present study is a pursuit to define implications of dual cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) (DuCLOX-2/5) inhibition on various aspects of cancer augmentation and chemoprevention. The monotherapy and combination therapy of zaltoprofen (COX-2 inhibitor) and zileuton (5-LOX inhibitor) were validated for their effect against methyl nitrosourea (MNU) induced mammary gland carcinoma in albino wistar rats. The combination therapy demarcated significant effect upon the cellular proliferation as evidenced through decreased in alveolar bud count and restoration of the histopathological architecture when compared to toxic control. DuCLOX-2/5 inhibition also upregulated levels of caspase-3 and caspase-8, and restored oxidative stress markers (GSH, TBARs, protein carbonyl, SOD and catalase). The immunoblotting and qRT-PCR studies revealed the participation of the mitochondrial mediated death apoptosis pathway along with favorable regulation of COX-2, 5-LOX. Aforementioned combination restored the metabolic changes to normal when scrutinized through 1H NMR studies. Henceforth, the DuCLOX-2/5 inhibition was recorded to import significant anticancer effects in comparison to either of the individual treatments.
Keywords: DuCLOX-2/5 inhibition; NMR; Zaltoprofen; Zileuton; angiogenesis; apoptosis; cyclooxygenase; lipoxygenase.
Figures


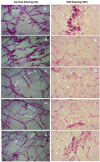
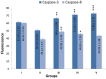


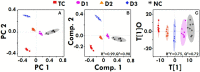

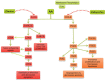
 denotes inhibition;
denotes inhibition;  denotes activation}.
denotes activation}.Similar articles
-
Effects of phenidone (DuCLOX-2/5 inhibitor) against N-methyl-N-nitrosourea induced mammary gland carcinoma in albino rats.Toxicol Appl Pharmacol. 2018 Jul 15;351:57-63. doi: 10.1016/j.taap.2018.04.019. Epub 2018 Apr 18. Toxicol Appl Pharmacol. 2018. PMID: 29679652
-
Repurposing mechanistic insight of PDE-5 inhibitor in cancer chemoprevention through mitochondrial-oxidative stress intervention and blockade of DuCLOX signalling.BMC Cancer. 2019 Oct 24;19(1):996. doi: 10.1186/s12885-019-6152-9. BMC Cancer. 2019. PMID: 31651285 Free PMC article.
-
DuCLOX-2/5 inhibition: a promising target for cancer chemoprevention.Breast Cancer. 2017 Mar;24(2):180-190. doi: 10.1007/s12282-016-0723-2. Epub 2016 Aug 24. Breast Cancer. 2017. PMID: 27558792 Review.
-
Rifaximin, a pregnane X receptor (PXR) activator regulates apoptosis in a murine model of breast cancer.RSC Adv. 2018 Jan 17;8(7):3512-3521. doi: 10.1039/c7ra09689e. eCollection 2018 Jan 16. RSC Adv. 2018. PMID: 35542911 Free PMC article.
-
The role of cyclooxygenase and lipoxygenase in cancer chemoprevention.Drug Metabol Drug Interact. 2000;17(1-4):109-57. doi: 10.1515/dmdi.2000.17.1-4.109. Drug Metabol Drug Interact. 2000. PMID: 11201293 Review.
Cited by
-
A site-moiety map and virtual screening approach for discovery of novel 5-LOX inhibitors.Sci Rep. 2020 Jun 29;10(1):10510. doi: 10.1038/s41598-020-67420-9. Sci Rep. 2020. PMID: 32601404 Free PMC article.
-
Chemoprophylactic activity of nitazoxanide in experimental model of mammary gland carcinoma in rats.3 Biotech. 2020 Aug;10(8):338. doi: 10.1007/s13205-020-02332-z. Epub 2020 Jul 8. 3 Biotech. 2020. PMID: 32670738 Free PMC article.
-
Repurposing Combination Therapy of Voacamine With Vincristine for Downregulation of Hypoxia-Inducible Factor-1α/Fatty Acid Synthase Co-axis and Prolyl Hydroxylase-2 Activation in ER+ Mammary Neoplasia.Front Cell Dev Biol. 2021 Nov 18;9:736910. doi: 10.3389/fcell.2021.736910. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34869321 Free PMC article.
-
Effect of Voacamine upon inhibition of hypoxia induced fatty acid synthesis in a rat model of methyln-nitrosourea induced mammary gland carcinoma.BMC Mol Cell Biol. 2021 Jun 5;22(1):33. doi: 10.1186/s12860-021-00371-9. BMC Mol Cell Biol. 2021. PMID: 34090331 Free PMC article.
-
PHD-2 activation: a novel strategy to control HIF-1α and mitochondrial stress to modulate mammary gland pathophysiology in ER+ subtype.Naunyn Schmiedebergs Arch Pharmacol. 2019 Oct;392(10):1239-1256. doi: 10.1007/s00210-019-01658-7. Epub 2019 Jun 1. Naunyn Schmiedebergs Arch Pharmacol. 2019. PMID: 31154466
References
-
- Ahmad Y., Sharma N. (2009). An effective method for the analysis of human plasma proteome using two-dimensional gel electrophoresis. J. Proteomics Bioinform. 2, 495–499. 10.4172/jpb.1000111 - DOI
-
- Belur B., Kandaswamy N., Mukherjee K. L. (1990). Laboratory Technology–A Procedure Manual for Routine Diagnostic Tests. New Delhi: Laboraoty Techniques in Histopathology; Tata McGraw Hill Co. Ltd.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

