Brain-Resident Microglia and Blood-Borne Macrophages Orchestrate Central Nervous System Inflammation in Neurodegenerative Disorders and Brain Cancer
- PMID: 29681904
- PMCID: PMC5897444
- DOI: 10.3389/fimmu.2018.00697
Brain-Resident Microglia and Blood-Borne Macrophages Orchestrate Central Nervous System Inflammation in Neurodegenerative Disorders and Brain Cancer
Abstract
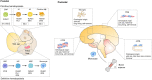
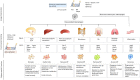
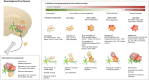
Inflammation is a hallmark of different central nervous system (CNS) pathologies. It has been linked to neurodegenerative disorders as well as primary and metastatic brain tumors. Microglia, the brain-resident immune cells, are emerging as a central player in regulating key pathways in CNS inflammation. Recent insights into neuroinflammation indicate that blood-borne immune cells represent an additional critical cellular component in mediating CNS inflammation. The lack of experimental systems that allow for discrimination between brain-resident and recruited myeloid cells has previously halted functional analysis of microglia and their blood-borne counterparts in brain malignancies. However, recent conceptual and technological advances, such as the generation of lineage tracing models and the identification of cell type-specific markers provide unprecedented opportunities to study the cellular functions of microglia and macrophages by functional interference. The use of different "omic" strategies as well as imaging techniques has significantly increased our knowledge of disease-associated gene signatures and effector functions under pathological conditions. In this review, recent developments in evaluating functions of brain-resident and recruited myeloid cells in neurodegenerative disorders and brain cancers will be discussed and unique or shared cellular traits of microglia and macrophages in different CNS disorders will be highlighted. Insight from these studies will shape our understanding of disease- and cell-type-specific effector functions of microglia or macrophages and will open new avenues for therapeutic intervention that target aberrant functions of myeloid cells in CNS pathologies.
Keywords: cancer; microglia; neurodegeneration; neuroinflammation; tissue-resident macrophages.
Figures

Similar articles
-
Sphingolipids and brain resident macrophages in neuroinflammation: an emerging aspect of nervous system pathology.Clin Dev Immunol. 2013;2013:309302. doi: 10.1155/2013/309302. Epub 2013 Sep 2. Clin Dev Immunol. 2013. PMID: 24078816 Free PMC article. Review.
-
Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration.Semin Immunopathol. 2013 Sep;35(5):601-12. doi: 10.1007/s00281-013-0382-8. Epub 2013 Jun 4. Semin Immunopathol. 2013. PMID: 23732506 Free PMC article. Review.
-
Differential transcriptional profiles identify microglial- and macrophage-specific gene markers expressed during virus-induced neuroinflammation.J Neuroinflammation. 2019 Jul 20;16(1):152. doi: 10.1186/s12974-019-1545-x. J Neuroinflammation. 2019. PMID: 31325960 Free PMC article.
-
Immune Microenvironment of Brain Metastases-Are Microglia and Other Brain Macrophages Little Helpers?Front Immunol. 2019 Aug 20;10:1941. doi: 10.3389/fimmu.2019.01941. eCollection 2019. Front Immunol. 2019. PMID: 31481958 Free PMC article. Review.
-
Usage of Multiparameter Flow Cytometry to Study Microglia and Macrophage Heterogeneity in the Central Nervous System During Neuroinflammation and Neurodegeneration.Methods Mol Biol. 2018;1745:167-177. doi: 10.1007/978-1-4939-7680-5_10. Methods Mol Biol. 2018. PMID: 29476469
Cited by
-
Single-Cell RNA Sequencing Reveals Immunomodulatory Effects of Stem Cell Factor and Granulocyte Colony-Stimulating Factor Treatment in the Brains of Aged APP/PS1 Mice.Biomolecules. 2024 Jul 10;14(7):827. doi: 10.3390/biom14070827. Biomolecules. 2024. PMID: 39062541 Free PMC article.
-
Taming microglia: the promise of engineered microglia in treating neurological diseases.J Neuroinflammation. 2024 Jan 11;21(1):19. doi: 10.1186/s12974-024-03015-9. J Neuroinflammation. 2024. PMID: 38212785 Free PMC article. Review.
-
Microglia in neurodegenerative diseases.Neural Regen Res. 2021 Feb;16(2):270-280. doi: 10.4103/1673-5374.290881. Neural Regen Res. 2021. PMID: 32859774 Free PMC article. Review.
-
The Journey of Cancer Cells to the Brain: Challenges and Opportunities.Int J Mol Sci. 2023 Feb 14;24(4):3854. doi: 10.3390/ijms24043854. Int J Mol Sci. 2023. PMID: 36835266 Free PMC article. Review.
-
Advancing basic and translational research to deepen understanding of the molecular immune-mediated mechanisms regulating long-term persistence of HIV-1 in microglia in the adult human brain.J Leukoc Biol. 2022 Nov;112(5):1223-1231. doi: 10.1002/JLB.1MR0422-620R. Epub 2022 May 25. J Leukoc Biol. 2022. PMID: 35612272 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

