Nervous system development in the Pacific oyster, Crassostrea gigas (Mollusca: Bivalvia)
- PMID: 29681988
- PMCID: PMC5896133
- DOI: 10.1186/s12983-018-0259-8
Nervous system development in the Pacific oyster, Crassostrea gigas (Mollusca: Bivalvia)
Abstract
Background: Bivalves comprise a large, highly diverse taxon of invertebrate species. Developmental studies of neurogenesis among species of Bivalvia are limited. Due to a lack of neurogenesis information, it is difficult to infer a ground pattern for Bivalvia. To provide more comprehensive morphogenetic data on bivalve molluscs and relationships among molluscan clades, we investigated neurogenesis in the Pacific oyster, Crassostrea gigas, from the appearance of the first sensory cells to the formation of the larval ganglionic nervous system by co-immunocytochemistry of the neuronal markers FMRFamide or 5-HT and vesicular acetylcholine transporter (VAChT).
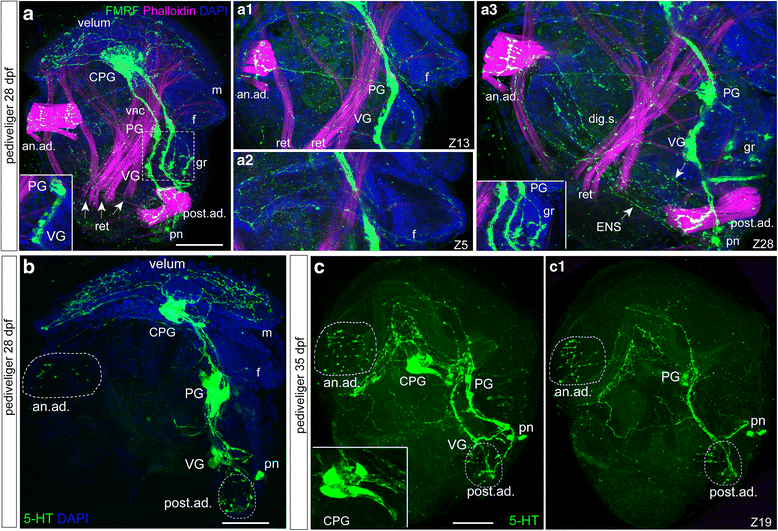
Results: Neurogenesis begins with the emergence of the apical serotonin-immunoreactive (5-HT-ir) sensory cells and paired sensory posttrochal dorsal and ventral FMRFamide-immunoreactive (FMRFamide-ir) cells at the early trochophore stage. Later, at the early veliger stage, the apical organ (AO) includes 5-HT-ir, FMRFamide-ir, and VAChT-ir cells. At the same stage, VAChT-ir cells appear in the posterior region of larvae and send axons towards the AO. Thus, FMRFamide-ir neurites and VAChT-ir processes form scaffolds for longitudinal neurite bundles develop into the paired ventral nerve cords (VNC). Later-appearing axons from the AO/CG neurons join the neurite bundles comprising the VNC. All larval ganglia appear along the VNC as paired or fused (epiathroid) clusters in late veliger and pediveliger larvae. We observed the transformation of the AO into the cerebral ganglia, which abundantly innervated the velum, and the transformation of ventral neurons into the pedal ganglia, innervating the foot, gills, and anterior adductor muscle. The visceral ganglia appear last in the pediveliger oyster and innervate the visceral mass and posterior adductor of premetamorphic larvae. In addition, a local FMRFamide-ir network was detected in the digestive system of pediveliger larvae. We identified VAChT-ir nervous elements in oyster larvae, which have not been observed previously in molluscs. Finally, we performed a morphology-based comparative analysis of neuronal structures among bivalve, conchiferan, and aculiferan species.
Conclusions: We described the development of the nervous system during the larval development in Crassostrea gigas. These data greatly advance the currently limited understanding of neurodevelopment in bivalves and mollusks, which has hampered the generation of a ground pattern reconstruction of the last common ancestor of Mollusca. Our morphological data support phylogenomic data indicating a closer Bivalvia-Gastropoda sister group relationship than the Bivalvia-Scaphopoda (Diasoma) group relationship.
Keywords: Acetylcholine; Evolution; FMRFamide; Larvae; Mollusca; Neurogenesis; Serotonin.
Conflict of interest statement
The field studies did not involve endangered or rare invertebrate species. No specific permissions were required to access the marine area, as it falls within Russian state-owned land.All authors agree on the submission and publication of this paper and its included figures and tables.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures







References
-
- Dame RF. Ecology of marine bivalves: an ecosystem approach. 2nd ed. New York: CRC press; 2011.
-
- Raineri M, Ospovat M. The initial development of gangliar rudiments in a posterior position in Mytilus galloprovincialis (Mollusca: Bivalvia) J Mar Biol Assoc UK. 1994;74:73–77. doi: 10.1017/S0025315400035670. - DOI
-
- Voronezhskaya EE, Nezlin LP, Odintsova NA, Plummer JT, Croll RP. Neuronal development in larval mussel Mytilus trossulus (Mollusca; Bivalvia) Zoomorphology. 2008;127:97–110. doi: 10.1007/s00435-007-0055-z. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

