High competing risks minimize real-world utility of adjuvant targeted therapy in renal cell carcinoma: a population-based analysis
- PMID: 29682181
- PMCID: PMC5908282
- DOI: 10.18632/oncotarget.24675
High competing risks minimize real-world utility of adjuvant targeted therapy in renal cell carcinoma: a population-based analysis
Abstract
Objective: To utilize a population-based approach to address the role of adjuvant TT in the management of RCC.
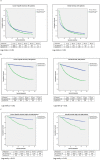
Methods: Patients with RCC (2006-2013) in the SEER database were stratified by metastatic disease at the time of diagnosis (cM0/cM1). cM0 patients following surgical excision were stratified into low and high-risk (ASSURE and S-TRAC criteria). Multivariable analyses performed to identify predictors of TT receipt; Fine and Gray competing risks analyses used to identify predictors of cancer-specific mortality (CSM). Subset analyses included patients with clear cell histology and high-risk cM0. Survival analyses were used to evaluate overall survival (OS) and cancer-specific survival (CSS) for all cohorts, stratified on TT receipt.
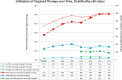
Results: 79,926 patients included (71,682 cM0, 8,244 cM1); median follow-up for the entire cohort was 40.1 months. Of 31,453 patients with histologic grade data, 18,328 and 13,125 were low- and high-risk cM0, respectively. TT utilization in cM1 patients peaked at 50.6% and was associated with reduced CSM (HR 0.73, p<0.01). In contrast, TT utilization (presumed salvage therapy) never exceeded 2.2% in the entire cM0 cohort and 3.5% in the high-risk cM0 cohort. On competing risks analysis, TT receipt was associated with increased CSM in all cohorts.
Conclusion: When compared to the cM1 patients, TT receipt in cM0 patients does not provide any cancer-specific survival benefit, even in the small percentage of patients that eventually progress to metastatic disease. Competing risks mortality further limit any potential benefit in this population. Based on current evidence, adjuvant TT cannot be recommended for RCC patients.
Keywords: carcinoma, renal cell; drug therapy; mortality; neoplasm metastasis; survival.
Conflict of interest statement
CONFLICTS OF INTEREST All authors report no conflicts of interest.
Figures
Similar articles
-
Percentage of sarcomatoid histology is associated with survival in renal cell carcinoma: Stratification and implications by clinical metastatic stage.Urol Oncol. 2022 Jul;40(7):347.e1-347.e8. doi: 10.1016/j.urolonc.2022.04.003. Epub 2022 May 9. Urol Oncol. 2022. PMID: 35551862
-
Real-World Survival Outcomes Associated With First-Line Immunotherapy, Targeted Therapy, and Combination Therapy for Metastatic Clear Cell Renal Cell Carcinoma.JAMA Netw Open. 2021 May 3;4(5):e2111329. doi: 10.1001/jamanetworkopen.2021.11329. JAMA Netw Open. 2021. PMID: 34032854 Free PMC article.
-
Survival outcomes and practice trends for off-label use of adjuvant targeted therapy in high-risk locoregional renal cell carcinoma.Urol Oncol. 2020 Jun;38(6):604.e1-604.e7. doi: 10.1016/j.urolonc.2020.02.028. Epub 2020 Mar 31. Urol Oncol. 2020. PMID: 32241693
-
Adjuvant therapy with tyrosine kinase inhibitors for localized and locally advanced renal cell carcinoma: an updated systematic review and meta-analysis.Urol Oncol. 2021 Nov;39(11):764-773. doi: 10.1016/j.urolonc.2021.07.022. Epub 2021 Aug 14. Urol Oncol. 2021. PMID: 34400065
-
Association between cytoreductive nephrectomy and survival among patients with metastatic renal cell carcinoma receiving modern therapies: a systematic review and meta-analysis examining effect modification according to systemic therapy approach.Cancer Causes Control. 2021 Jul;32(7):675-680. doi: 10.1007/s10552-021-01435-z. Epub 2021 May 8. Cancer Causes Control. 2021. PMID: 33963938
Cited by
-
Active surveillance in favorable intermediate-risk prostate cancer patients: Predictors of deferred intervention and treatment choice.Can Urol Assoc J. 2022 Jan;16(1):E7-E14. doi: 10.5489/cuaj.7272. Can Urol Assoc J. 2022. PMID: 34464250 Free PMC article.
-
Association of extended core sampling with delayed intervention and pathologic outcomes for active surveillance patients A population-based analysis.Can Urol Assoc J. 2024 May;18(5):E142-E151. doi: 10.5489/cuaj.8563. Can Urol Assoc J. 2024. PMID: 38319602 Free PMC article.
-
Risk of Secondary Malignancies After Pelvic Radiation: A Population-based Analysis.Eur Urol Open Sci. 2024 Mar 23;63:52-61. doi: 10.1016/j.euros.2024.02.013. eCollection 2024 May. Eur Urol Open Sci. 2024. PMID: 38558762 Free PMC article.
-
The clinical significance of epigenetic and RNAPII variabilities occurring in clear cell renal cell carcinoma as a potential prognostic marker.Transl Oncol. 2022 Jun;20:101420. doi: 10.1016/j.tranon.2022.101420. Epub 2022 Apr 10. Transl Oncol. 2022. PMID: 35417813 Free PMC article.
-
Upgrading on radical prostatectomy specimens of very low- and low-risk prostate cancer patients on active surveillance: A population-level analysis.Can Urol Assoc J. 2021 Jul;15(7):E335-E339. doi: 10.5489/cuaj.6868. Can Urol Assoc J. 2021. PMID: 33382372 Free PMC article.
References
-
- Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L, Merseburger AS, Mulders P, Powles T, Staehler M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–24. https://doi.org/10.1016/j.eururo.2015.01.005 - DOI - PubMed
-
- Motzer RJ, Jonasch E, Agarwal N, Beard C, Bhayani S, Bolger GB, Chang SS, Choueiri TK, Costello BA, Derweesh IH, Gupta S, Hancock SL, Kim JJ, et al. National comprehensive cancer network Kidney cancer, version 3.2015. J Natl Compr Canc Netw. 2015;13:151–59. https://doi.org/10.6004/jnccn.2015.0022 - DOI - PubMed
-
- Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G, Ginsberg MS, Bacik J, Kim ST, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–24. https://doi.org/10.1001/jama.295.21.2516 - DOI - PubMed
-
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, et al. TARGET Study Group Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. https://doi.org/10.1056/NEJMoa060655 - DOI - PubMed
-
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. https://doi.org/10.1056/NEJMoa065044 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

