Prognostic value of early 18F-FDG PET scanning evaluation in immunocompetent primary CNS lymphoma patients
- PMID: 29682187
- PMCID: PMC5908288
- DOI: 10.18632/oncotarget.24706
Prognostic value of early 18F-FDG PET scanning evaluation in immunocompetent primary CNS lymphoma patients
Abstract
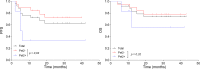
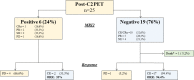
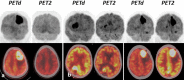
Primary central nervous system lymphoma (PCNSL) is a rare topographic variant of diffuse large B-cell lymphoma (DLBCL). While prognostic scales are useful in clinical trials, no dynamic prognostic marker is available in this disease. We report here the prognostic value of early metabolic response by 18F-FDG PET scanner (PET) in 25 newly diagnosed immunocompetent PCNSL patients. Induction treatment consisted of four cycles of Rituximab, Methotrexate and Temozolamide (RMT). Based on patient's general condition, consolidation by high-dose Etoposide and Aracytine was given to responding patients. Brain MRI and PET were performed at diagnosis, after two and four cycles of RMT, and after treatment completion. Two-year progression-free (PFS) and overall survival (OS) were 62% and 74%, respectively for the whole cohort. Best responses after RMT induction were 18 (72%) complete response (CR)/CR undetermined (CRu), 4 (16%) partial response, 1 (4%) progressive disease and 2 (8%) stable disease. Response evaluation was concordant between MRI and PET at the end of induction therapy. Nineteen patients (76%) had a negative PET2. Predictive positive and negative values of PET2 on end-of-treatment (ETR) CR were 66.67% and 94.74%, respectively. We observed a significant association between PET2 negativity and ETR (p = 0.001) and longer PFS (p = 0.02), while having no impact on OS (p = 0.32). Two years PFS was 72% and 33% for PET2- and PET2+ patients, respectively (p < 0.02). PET2 evaluation may help to early define a subgroup of CR PCNSL patients with a favorable outcome.
Keywords: PET scanner; primary CNS lymphoma.
Conflict of interest statement
CONFLICTS OF INTEREST The author(s) indicated no potential conflicts of interest.
Figures
References
-
- Shiels MS, Pfeiffer RM, Besson C, Clarke CA, Morton LM, Nogueira L, Pawlish K, Yanik EL, Suneja G, Engels EA. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br J Haematol. 2016;174:417–24. https://doi.org/10.1111/bjh.14073 - DOI - PMC - PubMed
-
- Hoang-Xuan K, Bessell E, Bromberg J, Hottinger AF, Preusser M, Rudà R, Schlegel U, Siegal T, Soussain C, Abacioglu U, Cassoux N, Deckert M, Dirven CMF, et al. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: guidelines from the European Association for Neuro-Oncology. Lancet Oncol. 2015;16:e322–32. https://doi.org/10.1016/S1470-2045(15)00076-5 - DOI - PubMed
-
- Omuro A, Correa DD, DeAngelis LM, Moskowitz CH, Matasar MJ, Kaley TJ, Gavrilovic IT, Nolan C, Pentsova E, Grommes CC, Panageas KS, Baser RE, Faivre G, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125:1403–10. https://doi.org/10.1182/blood-2014-10-604561 - DOI - PMC - PubMed
-
- Ferreri AJ, Cwynarski K, Pulczynski E, Ponzoni M, Deckert M, Politi LS, Torri V, Fox CP, Rosée PL, Schorb E, Ambrosetti A, Roth A, Hemmaway C, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3:e217–27. https://doi.org/10.1016/S2352-3026(16)00036-3 - DOI - PubMed
-
- Houillier C, Taillandier L, Lamy T, Chinot O, Molucon-Chabrot C, Soubeyran P, Gressin R, Choquet S, Damaj G, Thyss A, Jaccard A, Delwail V, Gyan E, et al. Whole Brain Radiotherapy (WBRT) Versus Intensive Chemotherapy with Haematopoietic Stem Cell Rescue (IC + HCR) for Primary Central Nervous System Lymphoma (PCNSL) in Young Patients: An Intergroup Anocef-Goelams Randomized Phase II Trial (PRECIS) Blood. 2016;128:782–782.
LinkOut - more resources
Full Text Sources
Other Literature Sources

